Ferrous Metals and Alloys
Ferrous Metals and Alloys
Chapter 5
Ferrous Metals and Alloys:
Production, General Properties, and Applications
5.1 Introduction
- Ferrous metals and alloys contain iron as their base metal, categorized as:
- Carbon and alloy steels
- Stainless steels
- Tool and die steels
- Cast iron
- Cast steels
- Ferrous alloys are produced for
- Sheet metal (automobiles, appliances, containers…).
- Structural members (bars, I beams, axles, railroads rails, …)
- Gears, tools, dies, molds
- Fasteners (bolts, rivets, nuts…)
- Typical US car contains about 800 kg of steel, accounting for 55-60% of its weight.
5.2 Production of Iron and Steels
5.2.1 Raw Materials
- Three basic materials used in iron and steel making:
- Iron Ore: about 5% of the earth’s crust. Principle ores are;
- Taconite (a black flint-like rock)
- Hematite (iron-oxide mineral)
- Limonite (iron oxide containing water)
- After it is mined, the ore is crushed into fine particles, the impurities are removed, and the ore is formed into pellets or balls using water and various binders.
- Typical pellets are about 65% pure iron and 25 mm in diameter.
- Coke: obtained from special grades of bituminous coal (soft coal rich in volatile hydrocarbons and tarry matter) that is heated in vertical ovens to temperature up to 1150oC and then cooled with water in quenching towers.
Coke has several functions in steelmaking:
- Generating high level of temp. needed for chemical reactions.
- Producing iron monoxide (a reducing gas; removes oxygen), which is then used to reduce iron oxide to iron.
- Limestone: (calcium carbonate) is used to remove impurities from the molten iron. Limestone reacts chemically with impurities, acting as a flux that causes impurities to melt at a low temperature. It combines with impurities and forms a slag (light) which floats over the molten metal, and, subsequently, is removed.
5.2.2 Ironmaking
- The three raw materials are carried out to the top of a blast furnace (Fig. 5.1) and dumped into it (charging the furnace). Furnace height is about ten-story building. Built in 1621 in US.
- The charge mixture is melted in a reaction at 1650oC, with air preheated to about 1100oC and blasted into the furnace through nozzles (called tuyeres).
- The basic reaction is that of oxygen with carbon to produce carbon monoxide which, in turn, reacts with iron oxide and reduces it to iron.
- Molten metal accumulates at the bottom of the blast furnace, while impurities float to the top of the metal.
- At intervals of 4-5 hours, the molten metal is drawn off (tapped) into ladle cars; as much as 160 tons of molten iron.
- This molten metal is called pig iron or simply hot metal; it has a typical composition of 4% C(Carbon), 1.5% Si(Silicon), 1% Mn(Manganese), 0.04% S(Sulfur), 0.4% P(Phosphorus), and the rest being pure iron.
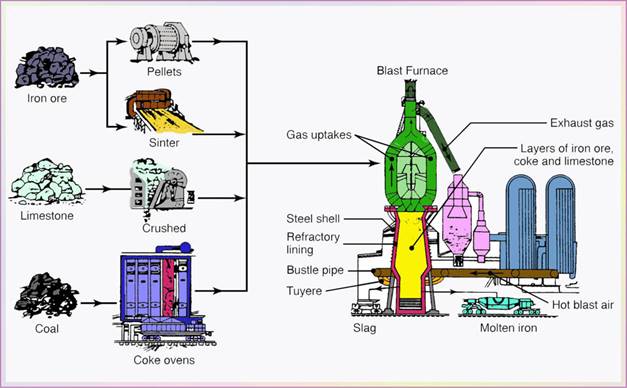 The solidified pig is then used in making iron and steels.
The solidified pig is then used in making iron and steels.
Figure 5.1 Schematic illustration of a blast furnace.
5.2.3 Steelmaking
- It is a process of refining the pig iron by the reduction of the percentage of manganese, silicon, carbon, and other elements and by controlling the composition of the output with the addition of various elements.
- Impurities are removed by re-melting the metal and adding carbon, steel scrap, and more limestone.
- The metal can be melted using one of three methods:
- Open-Hearth furnace
- Basic Oxygen furnace. (BOF)
- Electric furnace
- Open-Hearth furnace
- Uses a fuel to generate heat, and melt the metal.
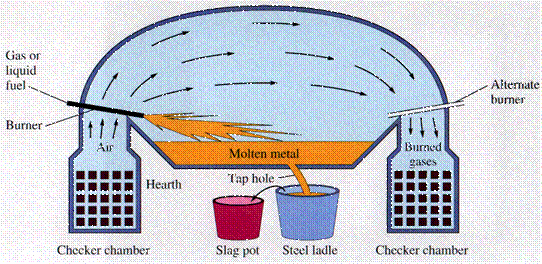
- Open-hearth has been replaced by electric furnaces and by the basic-oxygen process. The latter two are more efficient and produce better quality steels.
- Basic Oxygen Furnace (BOF)
- Fastest steelmaking process – can refine 250 tons of steel / hour.
- Melted pig iron (200 tons) and scrap (90 tons) are poured (charged) into a vessel (Fig. 5.3).
- Pure oxygen is then blown into the furnace for about 20 min through water-cooled lance (long tube), under a pressure of about 1250 kPa.
- Fluxing agents are added, like limestone.
- The vigorous agitation of the oxygen refines the molten metal by an oxidation process. This produces iron oxide which then reacts with carbon to produce CO and CO2.
- The lance is retracted, and the furnace is tapped by tilting it.
- The slag floats to the top of the metal and removed by tilting the furnace in the opposite direction.
- Higher steel quality than Open hearth. Used to make plates, sheets, I-beams, tubing and channels.
- Electric Furnace
- Main types of electric furnaces could be arc, induction, and resistance furnaces.
- A continuous electric arc formed between the electrodes and the charged metal (Fig. 5.2a and b) are the source of heat.
- Temperatures as high as 1925oC are generated.
- Usually 3 graphite electrodes are used, can be as large as 750 mm in diameter and 1.5-2 m in length. Their height inside the furnace can be adjusted depending on amount of metal present and the wear of the electrodes.
- Steel scrap and a small amount of carbon and limestone first are charged into the furnace.
- The roof is closed and the electrodes are lowered. The power then is turned on for about 2 hours; the metal melts.
- The current then is shut off, the electrodes are raised, the furnace is tilted, and the molten metal is poured into a ladle
- Can produce 60-90 tons of steel per day.
- Steel is higher quality than open-hearth and BOF.
- For smaller quantities, electric furnaces can be of the induction type: the metal is placed in a crucible surrounded with a copper coil through which alternating current is passed (Fig. 5.2c). The induced current in the charge generates heat and melts the metal.
- Vacuum furnaces are induction furnaces with air being removed, this removes the gaseous impurities from the molten metal and hence produces very high-quality steel.
5.3 Casting of Ingots
- While steel is still molten, it is poured from the ladle into molds in which the metal solidifies. The metal becomes an “ingot” in the mold.
- Ingots may be square, rectangle or round in cross section and their weight may range from a few hundred pounds to 40 tons.
- The cooled ingots are removed from the molds and lowered into soaking pits, where they are reheated uniformly to be rolled or formed into a final product.
- HOWEVER – While the molten metal cools, or solidifies, gasses evolve and can affect the quality of the steel. This leads to three types of steel: Killed Steel, Semi-Killed Steel, and Rimmed Steel.
- Fully deoxidized steel, oxygen is removed and porosity is eliminated. In the de-oxidation process, the dissolved oxygen reacts with elements such as Al, Si, Mn, and Vn. The impurities rise and mix with the slag.
- It is called killed because when the metal is poured it has no bubbles, it is quiet.
- The chemical and mechanical properties of killed steel are relatively uniform throughout.
- Because of shrinkage during solidification, a pipe or ‘shrinkage cavity’ is developed at the top of the ingot. This must be cut off and scrapped
- Partially deoxidized steel with some porosity (generally in the upper central section of the ingot)
- Little or no pipe; scrap is reduced
- Economical to produce
- Low carbon content (<0.15%)
- Evolved gases are killed partially using elements such as Al to the molten metal. The gasses then form blowholes around the rim of the ingot
- Little or no piping
- Ductile skin with good surface finish
- Impurities and inclusions tend to segregate toward the center of the ingot, so products made from this steel may be defective and should be inspected.
Refining:
- It is the removal of impurities.
- Most refining is done by adding various elements in the melting furnace or in ladles.
- It is important particularly in producing high-grade steels and alloys for high-performance applications.
- Secondary refining includes melting and processing the steel in a vacuum.
One system for strand continuous casting is shown schematically in Fig. 5.4:
- Molten metal in the ladle is cleaned, and then it is equalized in temperature by blowing nitrogen gas through it for 5-10 minutes. The metal then is poured into a refractory-kind pouring vessel (tundish) which holds as much as 3 tons of metals, where impurities are skimmed off.
- The molten metal travels downward through water cooled copper molds and begins to solidify into a path supported by pinch rollers that determine the final form (I-beams, channels..)
- When metal is first poured, it freezes onto the dummy bar which is withdrawn at the same rate at which the metal is poured.
- The metal descends @ about 1”/sec
- The solidified metal then goes through ‘pinch rollers’.
- Metal has more uniform composition and properties than ingot processing.
- Costs less to produce final product
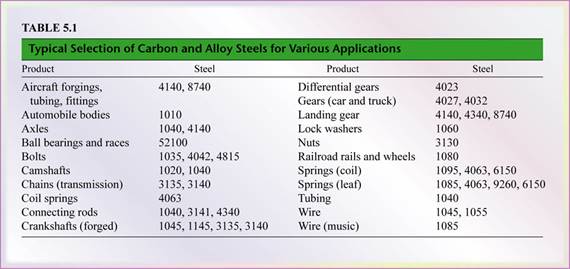
- Carbon and alloy steels are among the most commonly used metals and have a wide range of applications (see Table 5.1), depending on their composition and controlled processing.
- Basic product shapes include plate, sheet, strip, bar, wire, tube, castings, and forgings.
- Effects of Elements in Steels
- Different elements are added to steels to given properties such as hardenability, strength, hardness, toughness, wear resistance, workability, weldability, and machinability.
- Increasing the percentages of alloying elements in steels, increases the properties they impart.
- Some properties are beneficial while others are detrimental.
- Boron: Improves hardenability without the loss of (or even with some improvement in) machinability and formability.
- Calcium: Deoxidizes steels, improves toughness, and may improve formability and machinability.
- Carbon: improves hardenability, strength, hardness, and wear resistance; it reduces ductility, weldability, and toughness.
- Cerium: controls the shape of inclusions and improves toughness in high-strength low alloy steels; it deoxidizes steels.
- Chromium: improves toughness, hardenability, wear and corrosion resistance, and high-temperature strength; it increases the depth of the hardness penetration resulting from heat treatment by promoting carburization.
- Cobalt: improves strength and hardness at elevated temperatures.
- Copper: improves resistance to atmospheric corrosion and, to a lesser extent, increases strength with little loss in ductility; it adversely affects the hot-working characteristics and surface quality.
- Lead: improves machinability; it causes liquid-metal embrittlement.
- Magnesium: has the same effects as cerium.
- Manganese: improves hardenability, strength, abrasion resistance, and machinability; it deoxidizes the molten steel, reduce shot shortness, and decreases weldability.
- Molybdenum: improves hardenability, wear resistance, toughness, elevated-temperature strength, creep resistance, and hardness; it minimizes temper embrittlement.
- Nickel: improves strength, toughness, and corrosion resistance; it improves hardenability.
- Niobium (columbium): imparts fineness of grain size and improves strength and impact toughness; it lowers transition temperature and may decrease hardenability.
- Phosphorus: improves strength, hardenability, corrosion resistance, and machinability; it severely reduces ductility and toughness.
- Selenium: improves machinability.
- Silicon: improves strength, hardness, corrosion resistance, and electrical conductivity; it decreases magnetic-hysteresis loss, machinability, and cold formability.
- Sulfur: Improves machinability when combined with manganese; it lowers impact strength and ductility and impairs surface quality and weldability.
- Tantalum: has effects similar to those of niobium.
- Tellurium: improves machinability, formability, and toughness.
- Titanium: improves hardenability; it deoxidizes steels.
- Tungsten: has the same effects as cobalt.
- Vanadium: improves strength, toughness, abrasion resistance, and hardness at elevated temperatures; it inhibits grain growth during heat treatment.
- Zirconium: has the same effects as cerium.
- Residual Elements in Steels
- During the processing of steels some residual elements remain in the metal.
- These residuals are trace elements that are unwanted due to their detrimental properties but cannot be extracted completely:
- Antimony and Arsenic: cause temper embrittlement
- Hydrogen: severely embrittles steels, however, heating during processing drives out most of hydrogen.
- Nitrogen: improves strength, hardness, and machinability. In Al deoxidized steels, it controls the size of inclusions and improves strength and toughness. It decreases ductility and toughness.
- Oxygen: increases strength of rimmed steel. It severely reduces toughness.
- Tin: causes hot shortness and temper embrittlement
- Designations for Steels (introduction)
- Traditionally, the American Iron and Steel Institute (AISI) and the Society of Automotive Engineering (SAE) have designated carbon and alloy steels by using 4 digits.
- First two digits: alloying elements and their %weight
- Last two digits: carbon content %weight.
- Another numbering system has been the American Society for Testing and Materials (ASTM) which combines the AISI and SAE designations.
- The present numbering system is known as the Unified Numbering System (UNS) and has been adopted widely by ferrous and nonferrous industries. It consists of a letter, followed by five digits designating it chemical composition
- G – for AISI and SAE carbon and alloy steels
- J – for cast steels
- K – for miscellaneous steels and ferrous alloys
- S – for stainless steels and super alloys
- T – for tool steels
Example: G41300 for AISI 4130 alloy steel.
0-SAE steel grades
2-AISI-SAE Steel Alloy Designations Table
3-The AISI-SAE Steel Designation
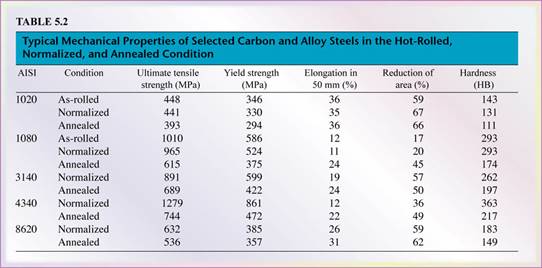
- Carbon steels are group by their percentage (by weight) of carbon content. The higher the carbon content the greater the hardness, strength and wear resistance after heat treatment.
- Low-carbon steel (mild steels) has < 0.30% carbon. Used in everyday industrial products like bolts, nuts, sheet, plate and tubes.
- Medium-carbon steel has 0.30% - 0.60% carbon. Used for jobs requiring higher strength such as machinery, automotive equipment parts (gears, axles,..), and metalworking equipments.
- High-carbon steel has > 0.60% carbon. Used parts that require the highest strength, hardness, and wear resistance such as cutting tools, cables, springs, etc. Once manufactured they are heat treated and tempered.
- Alloy steels are steels that contain significant amounts of alloying elements.
- Usually made with more care than carbon steels. Can be heat treated to obtain the desired properties.
- High-strength low-alloy steels (HSLA)
- Steels were developed to improve the strength-to-weight ratio.
- Low carbon content < 0.3%. Microstructure consists of fine grain ferrite as one phase and a hard 2nd phase of martensite and austenite.
- HSLA steels usually produced in sheet form commonly used in automobile bodies and in the transportation industry (the reduced weight makes for better fuel economy).
- Designations: 3 categories compose the AISI-designation system for high-strength sheet steel (Table 5.3).
- Structural quality (S): includes C, Mn, P, N.
- Low Alloys (X): contain Nb, Cr, Cu, Mn, Ni, Si, Ti, V, Zr
- Weathering steels (W): environmental corrosion resistance; Si, P, Cu, Ni, Cr.
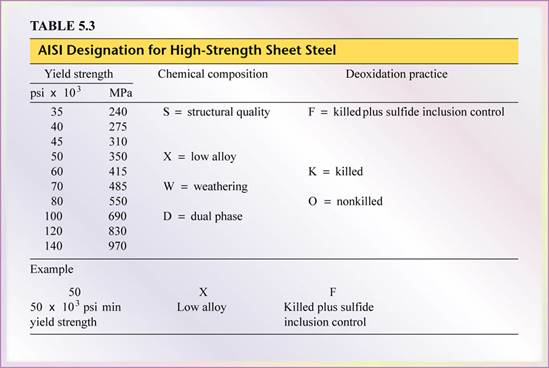
Note:
The formability of these sheets steels is grades by the letters F(excellent), K (good), O (fair).
4-High-strength low-alloy steel
- Microalloyed steels
- Recently developed HSLA steels provide superior properties without the use of heat treatment.
- When subjected to a controlled cooling (usually in air) these steels develop improved and uniform strength.
- Nanoalloyed steels
- Now under development.
- Have extremely small grain size (10-100 nm). Since their synthesis is done at an atomic level their properties can be specifically controlled.
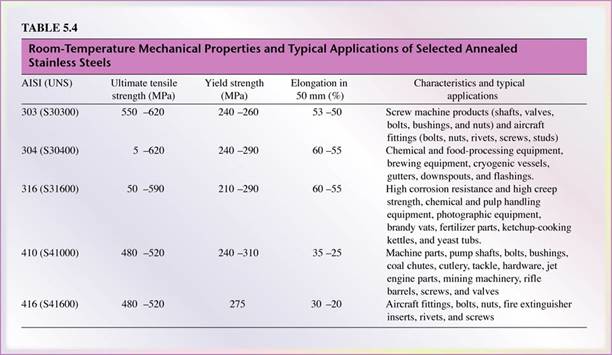
- Stainless steels are primarily known for their corrosion resistance, high strength, and ductility and chromium content.
- The reason for the name stainless is due to the fact that in the presence of oxygen, the steel develops a thin, hard, adherent film of chromium oxide and protects the metal from corrosion.
- Even if the surface is scratched, the protective film is rebuilt through passivation.
- For passivation to occur the minimum chromium content should be 10% to 12% by weight.
- Other alloying elements: Ni, Mo, Cu, Ti, Si, Mn, Al, N, and S.
- The higher the carbon content, the lower corrosion resistance of stainless steels. The reason is that the carbon reacts with chromium to form chromium carbide; hence reduces the content of chromium which lowers the passivity of the steel.
- Typical applications include cutlery, kitchen equipments, health care and surgical equipment, chemical industry, food processing, and petroleum industries.
- A recent trend is to use stainless steels as reinforcing bars in concrete structures such as highways, buildings, and bridges; especially in a marine environment. The advantages are better mechanical properties and corrosion resistance against chlorides from road salts and from the concrete itself.
- Five types: (Table 5.4)
- Austenitic (200 and 300 series) - composed of chromium, nickel, and manganese in iron. Nonmagnetic with excellent corrosion resistance; hardened by cold working; most ductile of all stainless steels.
- Ferritic (400 series) - high chromium content up to 27%. Magnetic with good corrosion resistance and lower ductility than austenitic stainless steel. Hardened by cold working.
- Martensitic (400 and 500 series) - Most martensitic stainless steel do not include nickel and are hardened by heat treatment. Chromium may be as much as 18% Magnetic with high strength, hardness, fatigue resistance, good ductility, and moderate corrosion resistance
- Precipitation hardening (PH) - Contain chromium and nickel along with copper, aluminum, titanium, or molybdenum. Good corrosion resistance and ductility, high strength at high temperatures. Aircraft and aerospace structural components.
- Duplex structure - Mixture of austenite and ferrite. Good strength and higher corrosion resistance than do the 300 series. Typical applications are water-treatment plants and heat-exchanger components.
0-SAE steel grades
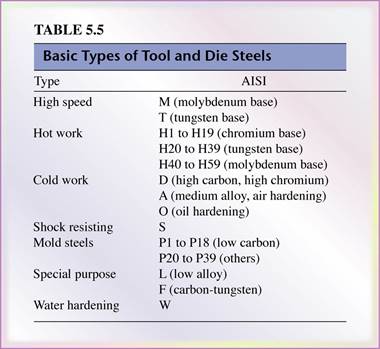
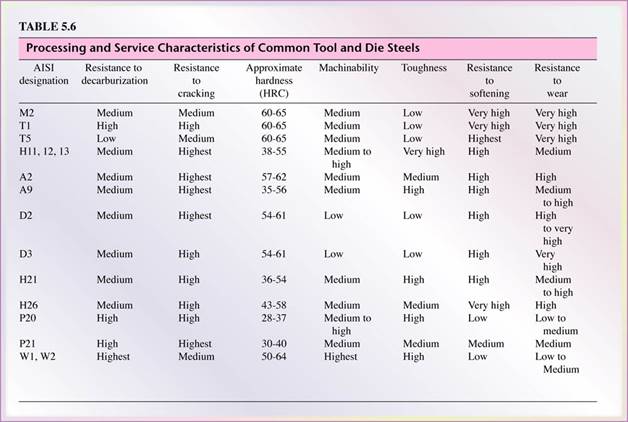
- Tool and die steels are alloyed steels (Tables 5.5 and 5.6) designed for high strength, impact toughness, and wear resistance at room and elevated temperatures.
- Commonly used in the forming (Dies) and machining (tools) of metals.
- High-speed steels (HSS) are the most highly alloyed tool and die steels.
- They maintain their hardness and strength at elevated operating temperatures.
- There are two basic types of HSS:
the molybdenum type (M-series) and the tungsten type (T-series).
- M-series steels contain up to about 10 % molybdenum with chromium, vanadium, tungsten, and cobalt as other alloying elements,
- T-series steels contain 12% to 18% tungsten with chromium, vanadium, and cobalt as other alloying elements.
- The M-series generally have higher abrasion resistance than T-series, undergo less distortion in heat treatment, and are less expensive.
- Hot-work steels (H-series) are designed for use at elevated temperatures. They have high toughness and high resistance to wear and cracking. The alloying elements are generally tungsten, molybdenum, chromium, and vanadium.
- Cold-work steels (A-, D-, O- series) are used for cold-working operations. They have high resistance to wear and cracking. These steels are available in oil-hardening or air-hardening types.
- Shock-resisting steels (S-series) are designed for impact toughness and are used in applications such as header dies, punches, and chisels (see Table 5.7).
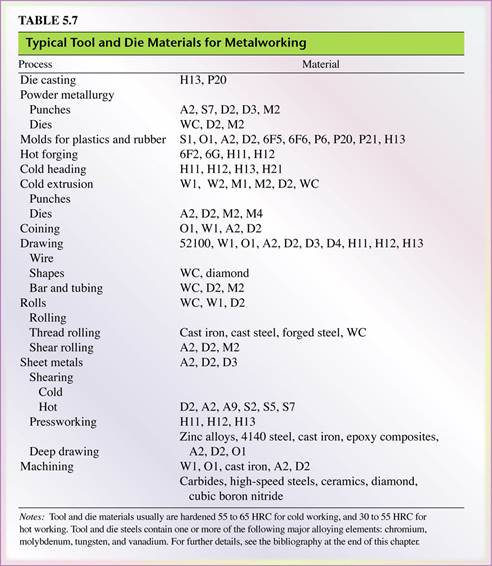
5.8 Cast Irons
- Cast irons are ferrous alloys composed of iron, carbon (2.11% - 4.5%), and silicon (up to 3.5%).
- They are classified, according to their solidification from the eutectic temperature, as follows:
- Gray cast iron, or gray iron.
- Ductile cast iron, nodular cast iron, or spheroidal graphite cast iron.
- White cast iron.
- Malleable iron.
- Compacted graphite iron.
- Cast irons are also classified by their structure: ferrite, pearlite, quenched and tempered, or austempered.
Source: http://site.iugaza.edu.ps/jelzebda/files/2014/02/Ch051.doc
Web site to visit: http://site.iugaza.edu.ps
Author of the text: indicated on the source document of the above text
If you are the author of the text above and you not agree to share your knowledge for teaching, research, scholarship (for fair use as indicated in the United States copyrigh low) please send us an e-mail and we will remove your text quickly.
Fair use is a limitation and exception to the exclusive right granted by copyright law to the author of a creative work. In United States copyright law, fair use is a doctrine that permits limited use of copyrighted material without acquiring permission from the rights holders. Examples of fair use include commentary, search engines, criticism, news reporting, research, teaching, library archiving and scholarship. It provides for the legal, unlicensed citation or incorporation of copyrighted material in another author's work under a four-factor balancing test. (source: http://en.wikipedia.org/wiki/Fair_use)
The information of medicine and health contained in the site are of a general nature and purpose which is purely informative and for this reason may not replace in any case, the council of a doctor or a qualified entity legally to the profession.
Ferrous Metals and Alloys
The texts are the property of their respective authors and we thank them for giving us the opportunity to share for free to students, teachers and users of the Web their texts will used only for illustrative educational and scientific purposes only.
All the information in our site are given for nonprofit educational purposes
Ferrous Metals and Alloys
 The solidified pig is then used in making iron and steels.
The solidified pig is then used in making iron and steels.







