GALVANIC CATHODIC BLISTERING.
Introduction
Carbon fiber reinforced polymer composites were shown to be susceptible to damage when galvanicaly coupled to active metals such as aluminum alloys and steels in seawater. It should be remembered that carbon is electrochemically active and will support reactions on its surface, mainly the cathodic reduction reaction of dissolved oxygen to form hydroxyl ions. The other side of the half cell is the anodic oxidation of the active metal, such as aluminum or iron in steel. One result of this galvanic connection is that the anodic or oxidation rate for the metal is increased by coupling to carbon fibers. The increase of the cathodic rate also has important consequences for the polymer based carbon fiber. These will be discussed in the next section.

In the figure above a portion of a carbon fiber mast is shown, with metal couplings attached to it.
Damage forms.
In the first case the surface structure of the polymer based carbon fiber composite prior to exposure should be examined. A schematic diagram is shown below. The surface immediately exposed to solution will be the polymer layer over the carbon fibers. The carbon fibers will be the layer below. This can be simplified to a permeable membrane covering an electrochemically active electrode. The reaction taking place on the surface of the electrode will be the reduction of dissolved oxygen in water to produce hydroxyl ions. However the reaction rate in this case is initially controlled by the diffusion rate of the reactive components through the polymer to the carbon fiber surface. The rate is controlled by the type, thickness and quality of the polymer layer and the solution chemistry. Another factor is the retained moisture in the polymer after manufacture. Once the fibers are connected to the anode then the cathodic and anodic reactions initiate. The hydroxyl ions build up on the carbon fibers and react with components in the solution such as sodium ions to balance the electrical charge. This sets up an osmotic condition as the pressure from the sodium hydroxide increases. The reaction of the composite to the osmotic pressure determines the damage done to polymer layer.
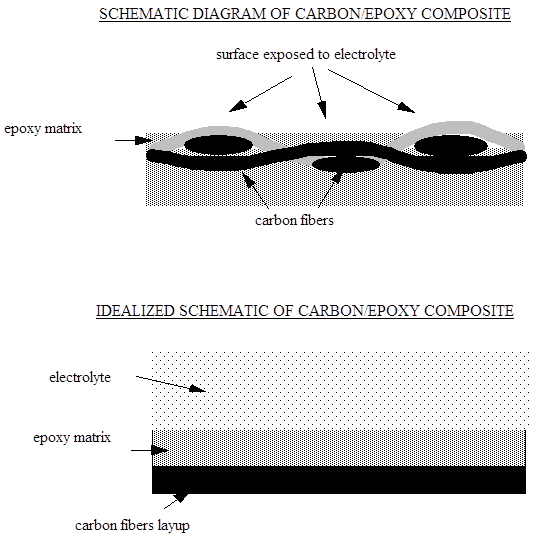
1. Thin polymer layer.
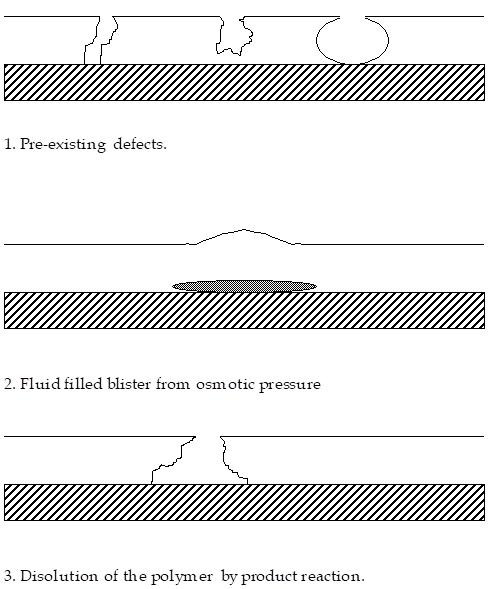
If the polymer layer over the location of osmotic pressure build up is thin, then the film will rupture. As a result, the solution will be directly exposed to carbon fibers with no intervening polymer layer. The current density and hence corrosion rate for the active species can then increase rapidly. The process is shown below.

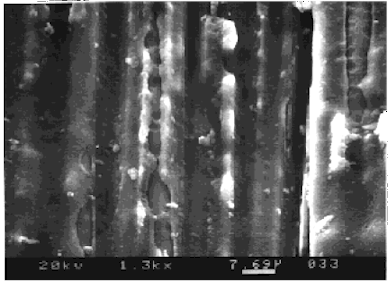
In the figure above, small holes are present where micro-blisters have burst due to osmotic pressure. Another form of damage created by the same process is shown in the next figure. Instead of individual blisters forming on the single fibers, splits or cracks were initiated, possibly due to a weak interface at the polymer to fiber interface.
In both these figures the diameter of a single carbon was around 8mm, so the marker bar in each case is about the diameter of a single carbon fiber in the image.
2. Thick polymer layer (100mm)
If the polymer is thick, then a different response is possible. As the osmotic pressure increases at the carbon fiber interface some depth below the surface, the stress level in the separating film does not increase very rapidly. The polymer can creep and slowly form a blister on the surface. The blister may or may not rupture. This appears to be a complex interrelation between the polymer thickness, strength and reaction rate.
3. Thick Polymer films
As the film thickens, the pressure or stress to form a blister is too large to overcome the creep strength of the polymer A surface blister will not form , but interfacial decohesion at the fiber polymer interface will still be present.

The surface of a carbon fiber composite is shown above prior to any exposure. In this case the composite was a hand lay up using carbon fiber mats made from tows. A tow is a bundle of many individual carbon fibers. The tows are running horizontally in the figure above and were about 0.125in in width. Small surface defects from the manufacturing process and be seen on the surface as small pores. The undulating weave of the carbon fibers can also be observed.

After exposure, the white areas in the figure above are delaminations where the fiber approach closest to the exposed surface. These delaminations are 200 mm below the surface at the interface of the polymer layer and the fiber layer.
Another process possible to degrade polymer films is that the reaction products on the cathode surface can react with and dissolve the polymer film, a direct dissolution mechanism shown below. This is a possible candidate.
At present , this process is very much in the research stage and so the mechanisms are very speculative.
Prevention.
1. Design
Design the composite structure so that any fasteners will not be in contact with carbon fibers. For examples around fixing holes in a composite structure use glass fibers which are non conductive rather than carbon fibers. The composite section may have to be increased due to lower strength.
2. Material Selection.
To date, an active metal would appear to be an important part of the process. Non active metals would decrease the rate of hydroxyl ion production. Unfortunately some of the alternatives are heavy and non really practical. Titanium may be useful.

Source: http://che.uri.edu/course/CHE534w/Chap%2010.doc
Web site to visit: http://che.uri.edu/
Author of the text: indicated on the source document of the above text
If you are the author of the text above and you not agree to share your knowledge for teaching, research, scholarship (for fair use as indicated in the United States copyrigh low) please send us an e-mail and we will remove your text quickly. Fair use is a limitation and exception to the exclusive right granted by copyright law to the author of a creative work. In United States copyright law, fair use is a doctrine that permits limited use of copyrighted material without acquiring permission from the rights holders. Examples of fair use include commentary, search engines, criticism, news reporting, research, teaching, library archiving and scholarship. It provides for the legal, unlicensed citation or incorporation of copyrighted material in another author's work under a four-factor balancing test. (source: http://en.wikipedia.org/wiki/Fair_use)
The information of medicine and health contained in the site are of a general nature and purpose which is purely informative and for this reason may not replace in any case, the council of a doctor or a qualified entity legally to the profession.
The texts are the property of their respective authors and we thank them for giving us the opportunity to share for free to students, teachers and users of the Web their texts will used only for illustrative educational and scientific purposes only.
All the information in our site are given for nonprofit educational purposes