Steel has many good properties, such as its high strength and toughness and the fact that it has good ductility and weldability.
One drawback however, is that it corrodes – meaning that its surfaces are prone to attack from environmental influences. Hence the attempts to eliminate this drawback by means of a surface coating. As well as organic paint coatings, metallic coatings are applied which are impermeable to practically all liquid and gaseous media.
The following requirements are made of metallic coatings:
Both of these requirements are fulfilled by zinc, to a large extent. This is why galvanisation (i.e. zinc coating) is such a commonly used method of corrosion protection. The automobile industry, too has tried to solve the corrosion problem by using galvanised sheets.
1.0 Galvanised Coatings
Its use in motor manufacturing is to protect the vehicle from rusting. Zinc has the required two-fold corrosion-inhibiting effect:
Zinc is an unstable metal that rapidly becomes covered with an oxide film on exposure to air. These zinc-oxide layers undergo a further reaction when exposed to the atmosphere and it is this that gives zinc its corrosion-inhibiting effect.
If two different metals are immersed in an electrically conductive fluid (= electroyte), an electric voltage is created between the two metals. If both metals are conductively inter-connected, an electric current will flow as a result of a flow of ions in the electrolyte. In this process, the ions of the less noble metal will dissolve and migrate to the more noble metal. The more noble metal will thus be completely preserved until such time as the less noble metal has been entirely used up. In the case of iron and zinc, it is zinc that is the less noble metal. In the event of mechanical damage to the surface of galvanised steel sheets, it is the resulting zinc layer that has to be scraped off first, before any corrosion attack on the steel itself is possible.
1.1 Welding Galvanised steel
The melting point of zinc is approx 420ºC and it vaporises at 906ºC. The temperature of a MAG arc, on the other hand, is between approx 3000ºC and 4000ºC.
As soon as the arc is ignited, zinc starts to vaporize in the arc-affected zone. These zinc vapours cause major turbulence in the shielding gas atmosphere, are conductive to spattering and can also lead to porosity.
The zinc vapours are damaged to health and must be extracted. Inhalation of zinc vapours leads to attacks of fever that will generally last for between one and two days (zinc fever).
Health and Safety, when Sanding and Welding Galvanised Material
Always use fume extraction Always use a respirator Always use appropriate pipe equipment
1.3 Galvanised Coatings on Vehicle Body Panels
Galvanised coatings are easily identified by its grey silvery colour. All manufacturers use it to protect the (ULASB) Ultra Light all Steel Body.
1.4 Manufacturing Methods
Light-gauge sheets and steel strip, as used in e.g. the automobile industry, are generally galvanised electrolytically. The thickness of the layer here is less than 10μm.
In this process, the parent sheet is drawn through an acid sulphate electroyle. In this case the sheet is the cathode and copper shoes act as the anode. The thickness of the layer is determined by the amperage and through-feed rate.
Production of a hot-galvanised light-gauge sheet
In this process, the parent sheet is drawn through a zinc bath with a temperature of approx. 500ºC. The desired layer-thickness is achieved by skimmer-jets that blow off excess zinc. In this process, galvanisation is only possible on both sides of the sheet simultaneously.
The thickness of the layer on the hot-galvanised sheets is given in g/m². The most commonly used layer thicknesses are:
The density of the zinc is approx. 7,15kg/dm³
Converting the layer thickness:
e.g. 275g/m² ÷ 7,15 = 38,46μ
Divided between 2 sides, meaning that the layer thickness is
19,23μ ~ 20 μ per side.
Note: The thickness limit for still-acceptable through-weldability of zinc layers may be regarded as approx. 20μ – although the position and type of seam play a crucial role.
1.5 Types of Galvanised Coating
The principal types of galvanisation are:
1.6 Levels of Galvanising
In hot-dip galvanisation, the components are immersed in a bath of molten zinc. The thickness of the zinc layer depends upon the immersion time, the composition of the steel and the temperature of the bath. As a rule, the zinc layer will be between 40 and 150μm thick (N.B 1 μm = 0,001mm). hot-dip galvanisation is normally performed after welding.
Zinc coating-thickness groups
The zinc coating thickness on electrolytically galvanised strip and sheet is given in μm per side. Internationally, the usual practise is that the designation gives 10 times the value of the rated coating thickness (ZE 10/10 =rated coating thickness 1 μm) on the top and the bottom of the sheet. Electrolytically galvanised strip and sheets can be supplied with the following coating thicknesses:
Fine coating ZE 10/10
Rated coating thickness of 1/1 μm, together with a suitable conservation coating, ensures corrosion protection for certain types of application.
Normal coating ZE 25/25
Rated coating thickness of 2,5/2,5 μm, together with a suitable conservation coating, ensures good corrosion protection.
Thicker coatings ZE 50/50 and ZE 75/75
Together with a suitable conservation coating, rated coating thicknesses of 5/5 μm (7,5/7,5 μm) ensure good corrosion protection and a stronger cathodic protective action at the cut edges than is the case with coatings of fine and normal thickness.
Single-side zinc coatings ZE 25/0,75/0 and 100/0
Rated coating thicknesses 2,5/0 μm and 5/0 μm, 7,5/0 and 10/0 μm.
Different thicknesses of zinc coating:
ZE 50/25, ZE 75/25, ZE 75/50
2.0 Seam Preparation
2.1 Repair Procedures/Panel Replacement Procedures
As already mentioned, the vapourisation reactions of the zinc may lead to the formation of pores. In overlapped joints, where the sheets lie on top of one another in parallel and without a gap, the zinc vapour can only escape through the weld pool. If the melt solidifies, the zinc vapour remains in the weld pool, and so forms pores.
Where a de-gassing gap is deliberately left, pore formation can be reduced and even completely prevented. The same is true, of course, for other seam preparations.
2.2 Removing or Reducing of Galvanised Coating
When selecting equipment, an orbital sander is the most appropriate, because it will not damage the substrate.
Never use a grinder as it will damage the substrate.
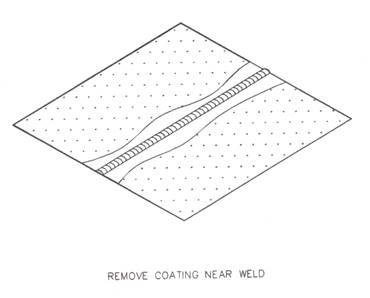
Figure 2 : Removing of Galvanised Coating
Summary
Sacrificial Protection:
This is the term used to describe the protection offered by zinc to the mild steel.
The automotive industry in seeking to provide extended warranties is turning increasingly to the use of zinc coated steels. Different areas of a vehicle require different zinc coatings and coatings weights to meet appearance and performance criteria.
zinc coating is the best way of protecting steel before it is pressed and formed into complex box sections, body sills, doors pillars and rook reinforcements, as access to these areas is difficult to spray paint.
Figure 4 : Door skin zinc coated on both sides
Source: http://local.ecollege.ie/Content/APPRENTICE/liu/vbr_notes/m1u7.doc
Web site to visit: http://local.ecollege.ie
Author of the text: indicated on the source document of the above text
If you are the author of the text above and you not agree to share your knowledge for teaching, research, scholarship (for fair use as indicated in the United States copyrigh low) please send us an e-mail and we will remove your text quickly. Fair use is a limitation and exception to the exclusive right granted by copyright law to the author of a creative work. In United States copyright law, fair use is a doctrine that permits limited use of copyrighted material without acquiring permission from the rights holders. Examples of fair use include commentary, search engines, criticism, news reporting, research, teaching, library archiving and scholarship. It provides for the legal, unlicensed citation or incorporation of copyrighted material in another author's work under a four-factor balancing test. (source: http://en.wikipedia.org/wiki/Fair_use)
The information of medicine and health contained in the site are of a general nature and purpose which is purely informative and for this reason may not replace in any case, the council of a doctor or a qualified entity legally to the profession.
The texts are the property of their respective authors and we thank them for giving us the opportunity to share for free to students, teachers and users of the Web their texts will used only for illustrative educational and scientific purposes only.
All the information in our site are given for nonprofit educational purposes