OBJECT:
To perform a Jominy end-quench test in order to observe heat treatment hardening and prepare the hardenability curve for a steel bar.
THEORY:
Steel is the most important engineering and construction material; it accounts for approximately 80 % of all metals produced. Steel has attained this degree of prominence because it combines strength, ease of fabricability into many shapes, and a wide range of properties along with low cost. Also it is possible to give a wide range of mechanical properties to steels by changing the size ad shape of the grains or changing its microconstituents. This property owes to several different ways that austenite can decompose.
Fundamentally, all steels are alloys of iron and carbon. So-called plain carbon steels also generally have small but specified amounts of phosphorus and sulfur. Alloy steels are those which contain specified percentages of other elements in their chemical compositions.
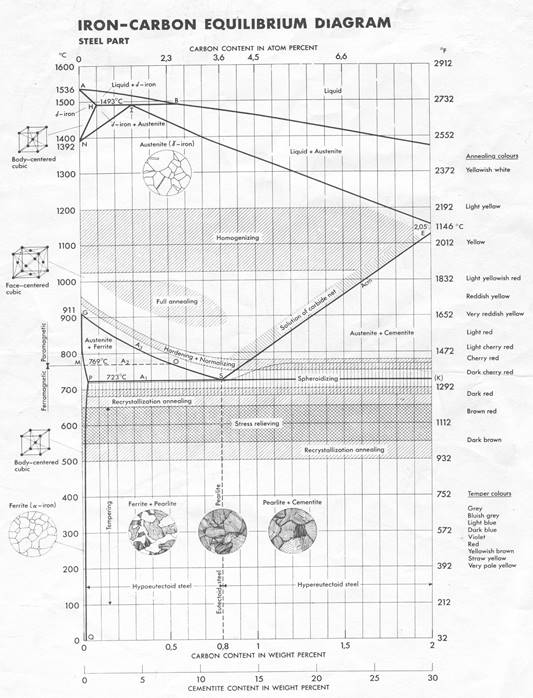
Figure 1. Iron-Carbon Equilibrium Diagram [1]
HARDENABILITY:
In general strength of a given steel is proportional to its hardness; the higher the hardness, the stronger the steel. The carbon content of a steel determines the maximum hardness attainable. The most important factor influencing the maximum hardness is mass of the metal being quenched. In a small section, the heat is extracted quickly, thus exceeding the critical cooling rate of the specific steel. The critical cooling rate is that rate of cooling which must be exceeded to prevent formation of non-martensite products.
Hardenability is the ease with which hardness may be attained. A steel that transforms rapidly from austenite to ferrite plus carbide has low hardenability because (a+carbide) is formed at the expense of martensite. Conversely a steel that transforms slowly from austenite to ferrite has greater hardenability.
For any given steel, there is a direct and consistent relationship between hardness and cooling rate. However the relationship is highly non-linear. There is a standardized test that lets us make necessary predictions of hardness. This is the Jominy end-quenched test. A round bar with a standard size is heated to form austenite and is than end-quenched with a water stream of specified flow rate and pressure. Hardness values along the bar are determined on a Rockwell harness tester and a Hardenability curve is plotted.
The quenched end is cooled very fast and therefore has the maximum possible hardness for the particular carbon content of the steel that is being tested. The cooling rates at points behind the quenched end are slower and consequently the hardness values are lower.
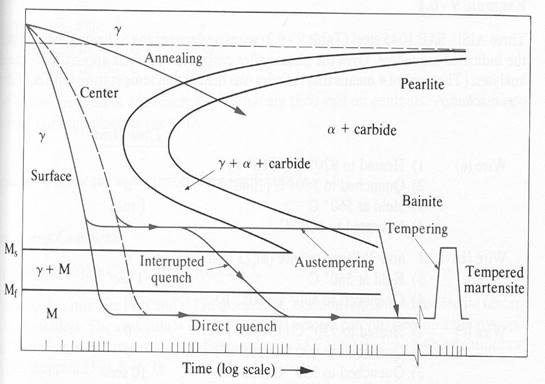
Figure 2. Transformation Processes [3].
HEAT TREATMENT:
The amount of carbon present in plain carbon steel has a pronounced effect on the properties of a steel and on the selection of suitable heat treatments to attain certain desired properties. Below are some major types of heat treatment processes:
Annealing: Steel is annealed to reduce the hardness, improve machinability, facilitate cold-working, produce a desired microstructure. Full annealing is the process of softening steel by a heating and cooling cycle, so that it may be bent or cut easily. In annealing, steel is heated above the transformation temperature to form austenite, and cooled very slowly, usually in the furnace.
There are several types of annealing like black annealing, blue annealing, box annealing, bright annealing, flame annealing, intermediate annealing, isothermal annealing, process annealing, recrystallisation annealing, soft annealing, finish annealing and spheroidizing. These are practiced according to their different final product properties in the industry.
The two-stage heat treating process of quenching and tempering is designed to produce high strength steel capable of resisting shock and deformation without breaking. On the other hand, the annealing process is intended to make steel easier to deform or machine. In manufacturing steel products, machining and severe bending operations are often employed. Even tempered steel may not cut or bend very easily and annealing is often necessary. Process annealing consists of heating steel to a temperature just below the A1 for a short time. This makes the steel easier to form. This heat treatment is commonly applied in the sheet and wire industries, and the temperatures generally used are from 1020 to 1200 0F (550 to 650 0C). Full annealing, where steel is heated 50 to 100 0F (90 to 180 0C) above the A3 for hypoeutectoid steels, and above the A1 for hypereutectoid steels, and slow cooled, makes the steel much easier to cut, as well as bend. In full annealing, cooling must take place very slowly so that a coarse pearlite is formed. Slow cooling is not essential for process annealing, since any cooling rate from temperatures below A1 will result in the same microstructure and hardness.
Normalizing: In normalizing steel is also heated above austenitizing temperature, but cooling is accomplished by still air cooling in a furnace. Steel is normalized to refine grain size, make its structure more uniform, or to improve machinability. When steel is heated to a high temperature, the carbon can readily diffuse throughout, and the result is a reasonably uniform composition from one area to the next. The steel is then more homogeneous and will respond to the heat treatment in a more uniform way.
The process might be more accurately described as a homogenizing or grain-refining treatment. Within any piece of steel, the composition is usually not uniform throughout. That is, one area may have more carbon than the area adjacent to it. These cornpositional differences affect the way in which the steel will respond to heat treatment. Because of characteristics inherent in cast steel, the normalizing treatment is more frequently applied to ingots prior to working, and to steel castings and forgings prior to hardening.
Hardening: Hardening is carried out by quenching a steel, that is cooling it rapidly from a temperature above the transformation temperature. Steel is quenched in water or brine for the most rapid cooling, in oil for some alloy steels, and in air for certain higher alloy steels. With this fast cooling rate, the transformation from austenite to pearlite cannot occur and the new phase obtained by quenching is called martensite. Martensite is a supersaturated metastable phase and have body centered tetragonal lettice (bct) instead of bcc. After steel is quenched, it is usually very hard and strong but brittle. Martensite looks needle-like under microscope due to its fine lamellar structure.
Tempering: Tempering (formerly called drawing), consists of reheating a quenched steel to a suitable temperature below the transformation temperature for an appropriate time and cooling back to room temperature. Freshly quenched martensite is hard but not ductile. Tempering is needed to impart ductility to martensite usually at a small sacrifice in strength.
The effect of tempering may be illustrated as follows. If the head of a hammer were quenched to a fully martensitic structure, it probably would crack after the first few blows. Tempering during manufacture of the hammer imparts shock resistance with only a slight decrease in hardness. Tempering is accomplished by heating a quenched part to some point below the transformation temperature, and holding it at this temperature for an hour or more, depending on its size.
The microstructural changes accompanying tempering include loss of acicular martensite pattern and the precipitation of tiny carbide particles. This microstructural is referred to as tempered martensite.
Stress Relieving: When a metal is heated, expansion occurs which is more or less proportional to the temperature rise. Upon cooling a metal, the reverse reaction takes place. That is, a contraction is observed. When a steel bar or plate is heated at one point more than at another, as in welding or during forging, internal stresses are set up. During heating, expansion of the heated area cannot take place unhindered, and it tends to deform. On cooling, contraction is prevented from taking place by the unyielding cold metal surrounding the heated area. The forces attempting to contract the metal are not relieved, and when the metal is cold again, the forces remain as internal stresses. Stresses also result from volume changes which accompany metal transformations and precipitation.
The term stress has wide usage in the metallurgical field. It is defıned simply as bad or force divided by the cross-sectional area of the part to which the bad or force is applied. Internal, or residual stresses, are bad because they may cause warping of steel parts when they are machined. To relieve these stresses, steel is heated to around 1100 0F (595 0C) assuring that the entire part is heated uniformly, then cooled slowly back to room temperature. This procedure is called stress relief annealing, or merely stress relieving.
ALLOYING ELEMENTS IN QUENCHING:
Because the sections treated are often relatively large and because the alloying elements have the general effect of lowering the temperature range at which martensite is formed, the thermal and transformational stresses set up during quenching tend to be greater in these alloy steel parts than those encountered in quenching the necessarily smaller sections of plain carbon steels. In general, the greater stresses result in distortion and risk of cracking.
Alloying elements, however, have two functions that tend to offset these disadvantages. First and probably most important is the capacity to permit use of a lower carbon content for a given application. The decrease in hardenability accompanying the decrease in carbon content may be readily offset by the hardenability effect of the added alloying elements and the lower carbon steel will exhibit a much lower susceptibility to quench cracking. This lower susceptibility results from greater plasticity of the low-carbon martensite and from the generally higher temperature range at which martensite is formed in the lower carbon materials. Quench cracking is seldom encountered in steels containing 0.25% carbon or less, and the susceptibility to cracking increases progressively with increasing carbon content.
The second function of the alloying elements in quenching is to permit slower rates of cooling for a given section, because of increased hardenability, thereby generally decreasing the thermal gradient and, in turn, the coolıng stress. It should be noted, however, that this is not altogether advantageous, since the direction, as well as the magnitude, of the stress existing after the quench is important in relation to cracking.
To prevent cracking, surface stresses after quenching should be either compressive or at a relatively low tensile level. In general, the use of a less drastic quench suited to the hardenability of the steel will result in lower distortion and greater freedom from cracking.
Furthermore the increased hardenability of these alloy steels may permit heat treatment by austempering or martempering, and thereby the level of adverse residual stress before tempering may be held to a minimum. In austempering, the workpiece is cooled rapidly to a temperature in the lower bainite region and is held at that temperature so that the section transforms completely to bainite. Because transformation occurs at a relatively high temperature and proceeds rather slowly, the stress level after transformation is quite low and distortion is minimal.
In martempering, the workpiece is cooled rapidly to a temperature just above Ms and held there until the piece attains a uniform temperature throughout, then cooled slowly (usually by air cooling through the martensite range. This procedure causes martensite to form more or less simultaneously throughout the entire section, thereby holding tranşformational stresses at a very low level, minimizing distortion and danger of cracking.
MECHANICAL PROPERTIES:
When quenched to martensite and tempered to the same hardness, carbon and alloy steels have similar tensile properties in that portion of the cross section that reacts to the quench. If carbon steel has the hardenability required by the critical section of the part and the quench used, the resulting tensile strength, yield strength and elongation in the fully hardened zone will be in the same range as in a similar zone in an alloy steel quenched and tempered to the same hardness. The similarity in properties of the hardened zone holds, regardless of the depth of hardening, but the strength of the piece will be governed by the thickness of the hardened zone (depth of hardening).
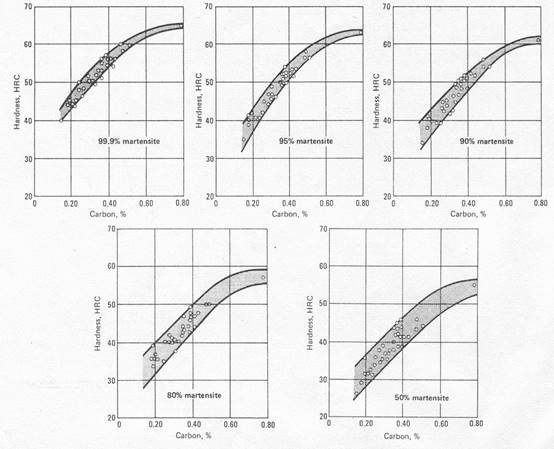
Figure 3. Effect of carbon on hardness of martensite structures [1].
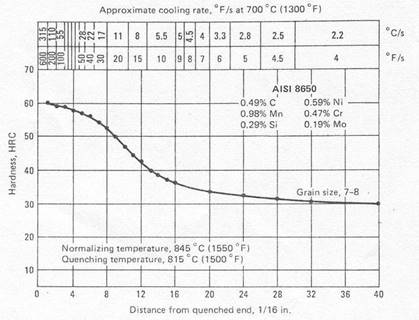
Figure 4. Method for presenting end-quench hardenability data [1].
One other and sometimes important difference between carbon and alloy steels is that, for the same hardness levels, fully quenched alloy steels require higher tempering temperatures than carbon steels. This higher tempering temperature is presumed to reduce the stress level in the finished parts without impairing mechanical properties.
Mechanical properties of carbon steels (particularly when quenched and tempered are influenced more by changes in section size than alloy steels because of the lower hardenability of carbon steels. In addition to the effect of section size on specific properties, the relation of one property to another is affected by size of the heat treated section. As the section size increases, incomplete response to hardening will lower the ratio of yield strength to tensile strength. The tensile strength decreases as the section size increases for a given composition and heat treatment, and there is some lowering of the ratio of yield to tensile strength.
EFFECT OF GRAIN SIZE:
The hardenability of a carbon steel may increase as much as 50% with an increase in austenite grain size from ASTM 8 (6 to 10) to ASTM 3 (1 to 4). The effect becomes more pronounced if the carbon content is increased at the same time. When the danger of quench cracking is remote (no abrupt changes in section thickness) and engineering considerations permit, it may sometimes appear to be more practical to use a coarser grained steel than a fine-grained on more expensive alloy steel to obtain hardenability. However, this is not recommended, because the use of coarser-grained steels usually involves a serious sacrifice in notch toughness and may lead to other difficulties.
HARDENABILITY TESTING:
Hardenability of a steel is best assessed by studying the hardening response of the steel to cooling in a standardized configuration in which a variety of cooling rates can be easily and consistently reproduced from one test to another.
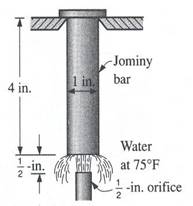
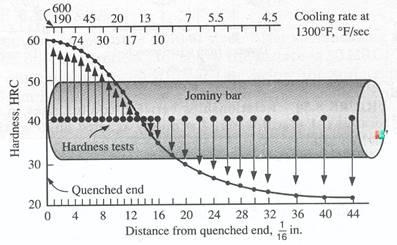
(a) (b) Figure 5. (a) Jominy end-quench hardenability test, (b) Typical distribution of hardness in Jominy bars.[4]
The end-quench, or Jominy, test: It fulfills the cooling rate requirements of hardenability testing most conveniently. The test specimen, a 1-in. (25.4 mm) dia. bar 4 in. (102 mm) in length, is water quenched on one end face. The bar from which the specimen is made must be normalized before the test specimen is machined. The test involves heating the test specimen to the proper austenitizing temperature and then transferring it to a quenching fixture so designed that the specimen is held vertically 12.7 mm above an opening through which a column of water may be directed against the bottom face of the specimen. While the bottom end is being quenched by the column of water, the opposite end is cooling slowly in air, and intermediate positions along the specimen are cooling at intermediate rates. After the specimen has been quenched, parallel flats 1800 apart are ground 0.015 in. (0.38 mm) deep on the cylindrical surface. Rockwell C hardness is measured at intervals of 1/16 in. (1.59 mm) for alloy steels and 1/32 in. (0.79 mm) for carbon steels, starting from the water-quenched end. Details of the standard test method are contained in specifications of the American Society for Testing and Materials (ASTM Method A255) and the Society of Automotive Engineers (Standard J406); in these specifications, dimensions are given in inches.
REFERENCES:
[1] Metals Handbook, ASM, Ohio, 1985
[2] Heat Treater’s Guide, Ed. Unterweiser, Boyer, Kubbs, ASM, Ohio, 1982
[3] Elements of Materials Science and Engineering, Lawrence H. Van Vlack, Addison-Wesley Publishing company, US, 1985
[4] Trojan F., Engineering Materials, 4. edition, Houghton Mifflin Company, New York, 1990, p:399.
Source: http://www.mslab.boun.edu.tr/Heat_treatment.doc
Web site to visit: http://www.mslab.boun.edu.tr/
Author of the text: indicated on the source document of the above text
If you are the author of the text above and you not agree to share your knowledge for teaching, research, scholarship (for fair use as indicated in the United States copyrigh low) please send us an e-mail and we will remove your text quickly. Fair use is a limitation and exception to the exclusive right granted by copyright law to the author of a creative work. In United States copyright law, fair use is a doctrine that permits limited use of copyrighted material without acquiring permission from the rights holders. Examples of fair use include commentary, search engines, criticism, news reporting, research, teaching, library archiving and scholarship. It provides for the legal, unlicensed citation or incorporation of copyrighted material in another author's work under a four-factor balancing test. (source: http://en.wikipedia.org/wiki/Fair_use)
The information of medicine and health contained in the site are of a general nature and purpose which is purely informative and for this reason may not replace in any case, the council of a doctor or a qualified entity legally to the profession.
The texts are the property of their respective authors and we thank them for giving us the opportunity to share for free to students, teachers and users of the Web their texts will used only for illustrative educational and scientific purposes only.
All the information in our site are given for nonprofit educational purposes