The basic functions of a lubricant are friction reduction, heat removal and suspension of contaminants. Designing a lubricant to perform these functions is a complex task, involving a careful balance of properties both in the base oil and the performance enhancing additives. This paper provides an overview of all the factors affecting proper lubricant performance, including a look at lubrication theory, base stock characteristics, additive design and function, and the needs of various end use applications.
Friction ReductionSimply stated, friction is reduced by maintaining a film of lubricant between surfaces that are moving with respect to each other, thereby preventing the surfaces from coming into contact and subsequently causing surface damage. Friction is a common element in daily life. One can walk up a steep ramp without slipping back because of high friction between shoe soles and the ramp surface. One can slide down a ski run because friction between packed snow and skis is low. Both cases illustrate friction between ordinary surfaces.
The coefficient is roughly constant for any pair of surfaces. For nonlubricated metal of ordinary surface finish and cleanliness, exposed to the atmosphere, the value may be about 1. For the same metal contaminated by handling, the value will drop to about 0.3 to 0.1. For well-designed and well-lubricated systems, the coefficient may be as low as 0.005. Under very special conditions, values as low as 0.000005 have been attained. By contrast, the coefficient for clean metal surfaces in a vacuum may be as high as 100 to 200 or more, and cold welding due to adhesion can occur.
Lubrication of a journal rotating in a cylindrical bearing offers the classic example of the hydrodynamic theory of bearing friction, as described by Osborne Reynolds in 1886. The theory assumes that under these conditions, friction occurs only within the fluid film, and is a function of fluid viscosity. Boundary Lubrication
Other viscosity systems, including Saybolt, Redwood, and Engler, have also been used because of their familiarity to many people. The instruments developed to measure viscosity in these systems are rarely used. Most viscosity determinations are made in centistokes and converted to values in other systems. |
Lubricant Base StocksA lubricant usually consists of a base fluid, generally of petroleum origin, combined with additive chemicals that enhance the various desirable properties of the base fluid. Base fluids are essentially obtained from two main sources: the refining of petroleum crude oil and the synthesis of relatively pure compounds with properties that are suitable for lubricants. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The boiling range of a compound increases roughly with an increase in the number of carbon atoms:
The heavier asphaltic materials cannot be vaporized because they decompose when heated above normal distillation temperatures, and their molecules either "crack" to form gas, gasoline and lighter fuels, or unite to form higher molecular weight molecules. These latter materials result in carbonaceous residues called "coke."
Synthetic Base Oils
These four types have found use in automotive lubricants, either alone or in combination with petroleum base oils. The table below lists some inspection characteristics of synthetic fluids together with the applicable temperature range.
Other fluids find niche uses in very specialized applications. These include polyglycols, phosphate esters, silicones, silicate esters, and polyphenyl ethers. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Some of the most important properties necessary for satisfactory lubricant performance are:
1. Low volatility under operating conditions. Volatility characteristics are essentially inherent in the choice of base oil for a particular type of service and cannot be improved by the use of additive materials.
2. Satisfactory flow characteristics in the temperature range of use. Flow characteristics largely depend on the choice of base oil; however, they can be improved through the use of pour point depressants and viscosity modifiers. The former improve low-temperature flow properties, while the latter enhance high-temperature viscosity characteristics.
3. Superior stability or ability to maintain desirable characteristics for a reasonable period of use. While these characteristics depend to some extent on the base oil, they are primarily associated with additive materials, which enhance base fluid properties in this area.
Lubricant stability is affected by the environment in which it operates. Such factors as temperature, oxidation potential and contamination with water, unburned fuel fragments, and corrosive acids limit the useful life of a lubricant. This is the area where additives have made a major contribution in improving the performance characteristics and extending the useful life of lubricants.
4. Compatibility with other materials in the system. Compatibility of lubricants with seals, bearings, clutch plates, etc., may also be partially associated with the base oil. However, additive chemistry can have a major influence on such characteristics.
Additives can be classified as materials that impart new properties to or enhance existing properties of the lubricant or fuel into which they are incorporated. It is not the object of this presentation to give a complete bibliography of the literature pertaining to these materials. An attempt will be made to present an overview of the field both as to chemistry and function.
The principal types of engine lubricant additives have been described in the literature by various authors. Materials of current interest in this area include:
Lubricant Additive Types
Detergents (Metallic Dispersants) |
Salicylates |
Ashless Dispersants |
N-substituted long-chain alkenyl succinimides |
Oxidation and Bearing Corrosion Inhibitors |
Organic phosphites |
Antioxidants |
Phenolic compounds |
Viscosity Modifiers |
Polymethacrylates |
Antiwear Additives |
Organic phosphites |
Pour Point Depressants |
Wax alkylated naphthalene |
*Also viscosity modifiers
Detergents
Materials of this type are generally molecules having a large hydrocarbon "tail" and a polar head group. The tail section, an oleophilic group, serves as a solubilizer in the base fluid, while the polar group is attracted to contaminants in the lubricant.
Although these compounds are commonly called detergents, their function appears to be the dispersing of particulate matter rather than cleaning up existing dirt and debris. Therefore, it is more appropriate to categorize them as dispersants. The molecular structure and a brief outline of the preparation methods for some representative types of metallic dispersants are discussed below.
Sulfonates
Sulfonates are the products of the neutralization of a sulfonic acid with a metallic base. The reaction can be illustrated as:
![]()
where MO = divalent metal oxide and MOH = divalent metal hydroxide. R represents the organic radical that acts as an oil solubilizing group.
The molecular weight of the hydrocarbon must be on the order of 350 or more, and the presence of the organic radical in the molecule is considered necessary for the oil solubility of the sulfonate. Commercially available sulfonates are of two types: petroleum sulfonates and synthetic sulfonates.
Petroleum (or natural) sulfonates are metal salts of sulfonic acids that were formerly byproducts of the sulfuric acid treatment of oil fractions in the manufacture of white oils. Currently, with the high demand for detergent oils, sulfonates rather than white oils have become the principal product. The structure of the organic portion of petroleum sulfonates is not completely known. Depending on the crude oil source, the structure can have varying proportions of aliphatic, naphthenic, and aromatic hydrocarbon groups.
Synthetic sulfonates are metal salts of acids produced from the sulfonation of alkylated aromatics by reaction with sulfur trioxide. In many cases, synthetic sulfonates were derivatives of benzene with long alkyl substituents, whose structure is illustrated at left, where R and R' are aliphatic radicals with a combined carbon number over C20.
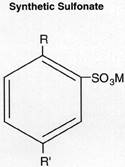
Most metallic cations of sulfonate detergents are calcium, magnesium, and sodium. Alkaline-earth sulfonates can be prepared by direct reaction of sulfonic acid with the metal oxide or hydroxide, or by reacting the sodium sulfonate with the metal chloride.
Oil-soluble sulfonates containing metal in excess of the stoichiometric amount are called basic sulfonates. Among the advantages of basic sulfonates is a greater ability to neutralize acidic bodies in addition to serving as a dispersant for contaminants.
Salicylates
Salicylates are generally prepared from alkyl phenols by a chemical scheme known as the Kolbe reaction.
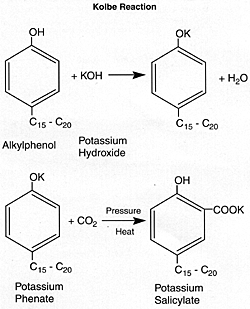
The potassium salicylate may be metathesized with calcium chloride or magnesium chloride. The resulting salts are then overbased to form highly basic detergents that have proven effective in diesel engine oil formulations.
Phenates and Phenol Sulfide Salts
The broad class of metal phenates includes the salts of alkylphenols, alkylphenol sulfides, and alkylphenol aldehyde products. Oil solubility is provided by alkylating the phenol with olefins that generally contain seven or more carbon atoms.
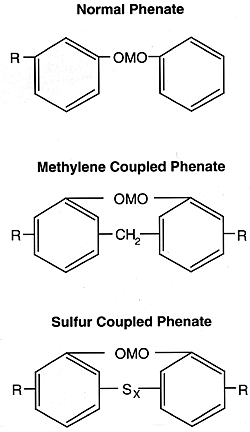
Sulfur is incorporated into the phenates by reacting the alkylphenol with sulfur chloride or elemental sulfur. The introduction of sulfur and the presence of a methylene bridge lowers the corrosivity of the products toward bearing materials and improves their antioxidant characteristics.
Calcium phenates are currently the most widely used types. They are manufactured by reacting the substituted phenols with the oxides or hydroxides of the metals. Basic phenates can be produced by using an excess of the metal base over the theoretical amounts required to form neutral phenates. Basic phenates have greater acid neutralization potential per unit of weight. Such products have two to three times the amount of metal required for neutral phenates.
In the structures for the various phenates shown, M = divalent metal and R = alkyl group.
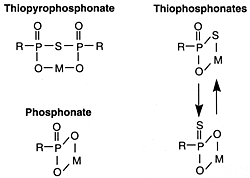
Thiophosphonates
Commercial products of this type are generally derived from acidic components produced by the reaction between polybutene (500 to 1000 molecular weight range) and phosphorus pentasulfide. A study of the structure of these compounds indicated that the organic salts present are principally thiopyrophosphonates, accompanied in some cases by 10 to 25 mole per cent of thiophosphonates and phosphonates. Oil-soluble phosphonates and thiophosphonates that contain metal in excess of the stoichiometric amount can also be prepared. However, these materials have almost vanished from use.
Dispersants
A major development in the additive field was the discovery and use of ashless dispersants. These materials may be categorized into two broad types: high-molecular weight polymeric dispersants used to formulate multigrade oils and lower molecular weight additives for use where viscosity modification is not necessary. These additives are much more effective than the metallic types in controlling sludge and varnish deposits that result from intermittent and low-temperature gasoline engine operation.
Compounds useful for this purpose are again characterized by a polar group attached to a relatively high molecular weight hydrocarbon chain. The polar group generally contains one or more of the elements nitrogen, oxygen and phosphorus. The solubilizing chains are generally higher in molecular weight than those in detergents; however, they may be quite similar in some instances.
No attempt will be made to describe all the materials that fit into this category. The discussion will be limited to some of the more widely used commercial products.
N-Substituted Long-Chain Alkenyl Succinimides
The majority of products currently in use are of this type or related materials that correspond to the following general formula:
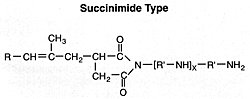
The alkenylsuccinic acid intermediate is obtained by condensing an olefin polymer, generally a polyisobutylene with a molecular weight in the range of 800 to 1200, with maleic anhydride. The basic part of the additive usually results from N-amino alkylpolyamines, especially the polyalkylene amines such as triethylenetetramine, tetraethylene pentamine, etc.
High Molecular Weight Esters
Materials of commercial interest in this area include products formed by the esterification of olefin substituted succinic acids with mono or polyhydric aliphatic alcohols. The olefin substituent in the acids has at least 50 aliphatic carbon atoms and a molecular weight of about 700 to 5000. An example of such a material is the reaction product of ethylene glycol with a substituted succinic anhydride:
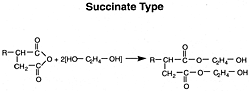
Polyhydric alcohols such as glycerol, pentaerythritol and sorbitol may be employed in such a reaction.
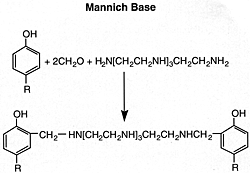
Mannich Bases from High Molecular Weight Alkylated Phenols
Such products are formed by the condensation of a high molecular weight alkyl-substituted phenol, an alkylenepolyamine, and an aldehyde such as formaldehyde. A description of the reaction product from polypropylenephenol, tetraethylenepentamine, and formaldehyde is:
Polymeric Dispersants
These ashless dispersants may serve the dual function of dispersant and viscosity modifier. They have two different structural features: those that are similar to materials employed as viscosity modifiers and those of polar compounds (which impart dispersancy). The viscosity modifiers will be discussed in a separate section. The general formula for dispersant polymers might be:
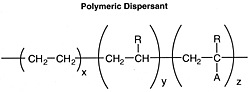
where the hydrocarbon portion is the oleophilic group, A = polar group, and R = hydrogen C1-6 alkyl, C4-6 alkenyl, or alkyl. Some of the many possibilities for the polar groups are:
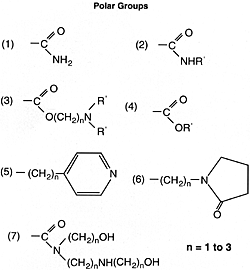
Oxidation and Bearing Corrosion Inhibitors
The function of an oxidation inhibitor is to prevent deterioration of the lubricant associated with oxygen attack. These inhibitors either destroy free radicals (chain breaking) or interact with peroxides involved in the oxidation mechanism. Among the widely used antioxidants are phenolic types and zinc dithiophosphates. The former are considered to be of the chain-breaking variety, whereas the latter are believed to be peroxide destroyers.
The corrosion of bearing metal is generally considered to be due largely to reaction of the acid with the oxides of the bearing metal. In engine operation, these acids either originate from products of incomplete fuel combustion that find their way into the lubricant as blowby gases or are produced from lubricant oxidation. Oxidation inhibitors can significantly reduce this tendency.
Detergents can reduce bearing corrosion by neutralizing the corrosive acids. Other inhibitors such as zinc dithiophosphates and phosphosulfurized olefins not only inhibit oxidation but also form a protective film on the bearing surface, making it impervious to acid attack.
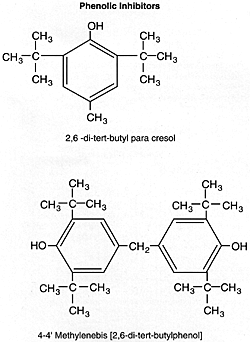
Phenolic Inhibitors (Chain-Breaking)
The inhibitor efficiency of phenol is markedly increased by substituting alkyl groups in the two ortho and para positions. It is particularly enhanced when the ortho substituents are bulky groups such as tert-butyl and the para substituent is a primarily alkyl group. A variety of hindered phenols are produced commercially and employed as inhibitors in transformer, turbine, and engine oils.
The methylenebis structure is more effective in high-temperature applications due to its lower volatility characteristics compared to the other molecule.
Zinc Dithiophosphates (Peroxide Destroying)
Of greatest commercial importance in engine lubricants are the zinc dithiophosphates, which not only serve as antioxidants but also provide both antiwear and bearing corrosion protection. The zinc dithiophosphates are made as follows:
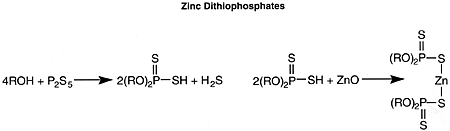
where R = alkyl or aryl. Both alkyl and aryl derivatives are employed commercially. Alkyl derivatives are generally more effective as antiwear additives. Aryl derivatives have a higher degree of thermal stability.
Both the antiwear and thermal stability characteristics of the alkyl compounds can be varied by using different alcohols; i.e. primary vs. secondary and high vs. low molecular weight. The principal alkyls are propyl, butyl, hexyl, octyl, and mixtures of these. The effect of the alkyl radical on the thermal decomposition temperature of zinc dialkyldithiophosphates (ZDP) is shown below:
Effect of Alkyl Radical on Thermal Decomposition of ZDP
Alkyl Radical |
Decomposition Temperature (°C) |
Isopropyl |
196 |
4-Methyl 2-pentyl |
197 |
N-Amyl |
212 |
N-Octyl |
>251 |
Stability increases with the length of the alkyl chain and is lower for secondary alkyl groups with the same number of carbon atoms. It should be noted, however, that the overall performance characteristics of ZDPs are not related to the decomposition temperature.
Antiwear Additives
Wear is the loss of metal with subsequent change in clearance between surfaces moving relative to each other. If continued, it will result in equipment malfunction. Among the principal factors causing wear are metal-to-metal contact, presence of abrasive particulate matter, and attack of corrosive acids.
Metal-to-metal contact can be prevented by adding film-forming compounds that protect the surface either by physical absorption or chemical reaction. The zinc dithiophosphates are widely used for this purpose and are particularly effective in reducing wear in valvetrain mechanisms. These compounds are described under oxidation and bearing corrosion inhibitors. Other effective additives contain phosphorus, sulfur, or combinations of these elements.
Abrasive wear can be prevented by effective removal of particulate matter by filtration of both the air entering the engine and the lubricant during engine operation.
Corrosive wear is largely the result of acidic blowby products formed during fuel combustion. This type of wear can be controlled by using alkaline additives such as basic phenates and sulfonates.
Viscosity Modifiers
Viscosity modifiers, or viscosity index improvers as they were formerly known, comprise a class of materials that improves the viscosity/temperature characteristics of the lubricant. This modification of rheological properties results in increased viscosity at all temperatures. The viscosity increase is more pronounced at high temperatures which significantly improves the viscosity index of the lubricant. This is manifested by a decrease in the slope of the viscosity/temperature line plotted on ASTM log paper.
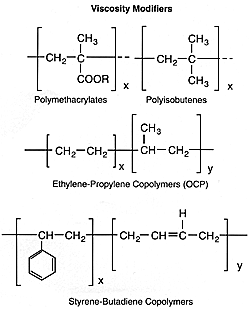
Viscosity modifiers are generally oil-soluble organic polymers with molecular weights ranging from about 10,000 to 1 million. The polymer molecule in solution is swollen by the lubricant, and the volume of the swollen entity determines the degree to which the polymer increases viscosity. The higher the temperature, the larger the volume and the greater the "thickening" effect of the polymer. Hence, the oil tends to "thin" less due to increased temperature.
In addition to viscosity improvement, the performance of these polymers also depends on shear stability or resistance to mechanical shear and on their chemical and thermal stability. With a given polymer system, shear stability decreases with an increase in molecular weight. The loss due to shear is reflected in a loss in lubricant viscosity. On the other hand, the "thickening power" of the viscosity modifier increases with an increase in molecular weight for a given polymer type. A performance balance must then be established which takes into consideration shear stability and viscosity needs as well as thermal and oxidative stability in actual engine operation.
Pour Point Depressants
Pour point depressants prevent the congelation of oil at low temperature. This phenomenon is associated with crystallization of the paraffin wax that is present in mineral oil fractions. To provide low pour points, the refiner removes wax constituents, which solidify at relatively high temperatures, in a process known as dewaxing. Complete dewaxing would reduce the yield of lube oil to an uneconomical level. Therefore, the dewaxing process is supplemented by using additives that lower the pour point of the oil.
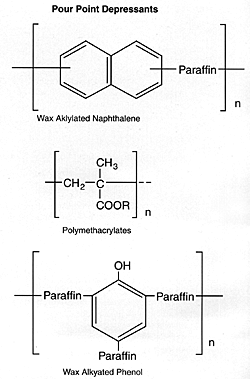
Pour point depressants do not prevent wax from crystallizing in the oil. Rather, they are absorbed on the wax crystals and, thus, reduce the amount of oil occluded on the crystal. Reducing the crystal volume permits lubricant flow.
Miscellaneous Additives
This category includes antirust compounds and foam inhibitors. Chemicals employed as rust inhibitors include sulfonates, alkenyl succinic acids, substituted imidazolines, amines, and amine phosphates. A considerable amount of information on these additives is contained in patent literature. Antifoam agents include silicones and miscellaneous organic copolymers.
While the general nature of additives may be the same for various types of lubricants, the specific types of additives chosen will depend upon the service in which the lubricant will be used and the characteristics of the base fluid. The development of a balanced additive package involves considerably more work than the casual use of each additive type mentioned. Extensive full-scale testing, both in the laboratory and in the field, is necessary to develop a suitable product.
Quite often, functional difficulties arise under actual operating conditions from combining these materials. On the other hand, unpredictable but desirable synergistic effects may also become evident. The only methods currently available to obtain such data are full-scale tests, some of which will be described below.
As noted previously, the principal functions of a lubricant are contaminant containment, heat removal and friction reduction. The important factors that influence these functions are:
Factors Influencing Lubricant Functions
Contaminant Containment |
1. Detergency — Dispersancy |
Heat Transfer |
1. Specific Heat |
Reduction of Frictional Effects |
1. Coefficient of Friction |
Note that thermal and oxidative stability are necessary to perform all functions.
Engine Lubricants
Most engines used in transportation are of the internal combustion type. These engines have high thermal efficiency and are lightweight relative to their power capability. The performance of engine lubricants is judged on their ability to reduce friction, resist oxidation, minimize deposit formation, and prevent corrosion and wear. To meet these functional requirements, engine lubricants must be supplemented with additives, as follows:
|
|
Most problems associated with engine lubricants are related to lubricant decomposition and the entry of combustion byproducts into the crankcase. The major causes of engine malfunction due to lubricant quality are deposit formation, contamination, oil thickening, oil consumption, ring sticking, corrosion, and wear.
Deposit Formation
The two main sources of lubricant contamination are blow-by from the combustion chamber, and gases and volatiles from the crankcase that are vented into the intake manifold as an antipollution measure. The various gases interact with one another and the lubricant to form soot, carbon, lacquer, varnish, and sludge.
Soot particles are hydrocarbon fragments partially stripped of hydrogen atoms. They also contain an appreciable amount of oxygen and sulfur. Soot particles are strongly attracted to one another and to polar compounds in the oil. Soot tends to form aggregates, which have a soft and flaky texture, and is commonly found in the combustion chamber.
Carbon deposits are hard and result from the carbonization of the liquid lubricating oil and fuel on hot surfaces. These deposits have a lower carbon content than soot and usually contain oily material and ash. They are commonly found on the piston top lands and crowns, in piston ring grooves, and on valve stems.
Lacquer and varnish form when oxygenated products in the lubricant are exposed to high temperatures. Lacquer is often derived from the lubricant and is generally water soluble. It is commonly found on pistons and cylinder walls and in the combustion chamber.
Varnish, on the other hand, is fuel related and is acetone soluble. It is commonly found on valve lifters, piston rings, and positive crankcase ventilation valves.
Sludge is caused by lubricant oxidation, oxidation and combustion products in the blow-by gas, and the accumulation of combustion water and dirt. It can vary in consistency from that of mayonnaise to a baked deposit. Low-temperature sludge, most prevalent in gasoline engines, is watery in appearance and forms below 95°C. High-temperature sludge is more common in diesel engines and forms above 120°C.
Oil Thickening
Oil thickening can result from lubricant oxidation, the accumulation of insolubles, and soot. Viscosity increases due to:
Oil Consumption
Oil consumption is related mainly to the lubricant that travels past piston rings and valves, and burns in the combustion chamber. The extent of lubricant consumption depends on a number of equipment and lubricant related factors, including viscosity, volatility and seal-swell characteristics.
A certain minimum amount of oil is required to properly lubricate the cylinder walls and pistons. High oil consumption, however, indicates a problem such as cylinder wear, bore polishing, stuck piston rings or out of square grooves. These conditions increase the amount of blow-by gases entering the crankcase.
Lubricant volatility is another important factor responsible for oil consumption. Lighter base oils can leak past the piston rings more readily and be burned.
Ring Sticking
The major cause of ring sticking is the formation of deposits in the piston grooves, resulting in the loss of an oil seal. This not only increases the potential for blow-by gases in the crankcase but also leads to poor heat transfer from the piston to the cylinder wall. Resultant thermal expansion of the pistons can lead to loss of compression and engine seizure.
Corrosion and Wear
Diesel fuel with high sulfur content causes piston ring and cylinder wear. Corrosive wear is more commonly associated with combustion and oxidation products; it results from the attack of sulfur acids or organic acids on iron surfaces. This kind of wear is controlled by using lubricants with a base reserve.
Engine Oil Performance
In the U.S., the classification of and requirements for engine oils are established through a process led by API, AAMA, EMA and CMA. These trade associations, together with ILMA, provide the framework for new engine oil categories. The technical societies ASTM and SAE verify the technical need for the new category and ultimately recommend the tests and performance limits to define the category.
In Europe, individual OEMs continue to be a major influence on engine oil performance requirements for both passenger-car and heavy-duty applications. The ACEA classification system consists of nine different sequences to define engine oil quality for European automotive service fill applications. The system is based on a schedule of physical, chemical and engine tests similar to those used in the U.S. but using both ASTM and CEC test methods. All performance claims against ACEA requirements must be supported by data generated under the European Engine Lubricant Quality Management System (EELQMS). This system consists of two codes of practice — one developed by ATC and one by ATIEL — and defines the process for developing, testing and reporting the necessary performance data.
In the U.S., API administers the licensing and certification of engine oils through a system that meets the warranty, maintenance and lubrication requirements of original equipment manufacturers (OEMs). Engine oil performance requirements, test methods, limits for the various classifications and testing processes are established cooperatively by the OEMs, oil marketers, additive companies and testing laboratories. These groups routinely review the classifications system and implement changes as conditions warrant. Click here to view current API Engine Service Classifications for gasoline and heavy-duty diesel engine oils, and the physical requirements for engine oils.
Gasoline Engine Lubrication
Lubrication problems in gasoline engines, particularly in passenger cars, are associated with:
Recognizing these problems, the automobile industry has sought to define the lubrication requirements of their engines in terms of engine dynamometer test procedures. Some of these test sequences are published by ASTM and involve:
In North America, all licensed oils must meet the requirements of ILSAC GF-2 and API SJ. The procedures for licensing and certifying engine oils by API are complemented by the Chemical Manufacturers Association (CMA) Product Approval Code of Practice, which provides a statistically valid testing and approval system for testing candidate oils. A key provision of the Code is multiple-test acceptance criteria for the Sequence IID, IIIE, VE, VI-A and L-38 tests.
In Europe, all licensed oils for gasoline powered engines must meet the requirements of ACEA A1, A2, or A3.
Diesel Engine Lubrication
Lubrication requirements for diesel engines are being driven by emission control requirements. New engine designs are being introduced to meet the latest emissions regulations, which are listed below. These changes subject the lubricant to more hostile operating conditions.
Year |
CO |
PM |
HC |
NOx |
1994 |
15.5 |
0.1 |
1.3 |
5.0 |
1998 |
15.5 |
0.1 |
1.3 |
4.0 |
2004 — Option 1 |
15.5 |
0.1 |
*NMHC + NOx =2.4 |
|
2004 — Option 2 |
15.5 |
0.1 |
*NMHC + NOx =2.5 |
|
*Nonmethane Hydrocarbons — cap of 0.5 g/hp-h in Option 2
In reducing emissions, the lubricant has been identified as a direct contributor to hydrocarbon particulates. This occurs partly from the leakage of lubricant past exhaust valve guides and turbocharger seals, but mainly from consumption of the lubricant film on the cylinder liner during combustion. To reduce particulate emissions, new engines are designed to operate with a thinner lubricant film and to allow less lubricant leakage past the piston rings. Unfortunately, this can increase the potential for ring and liner scuffing, and can increase operating temperatures and the potential for deposit formation. In addition, the engine modifications and adjustments necessary to ensure compliance with 1994 exhaust emissions regulations also had the adverse effect of increasing lubricant soot levels, which can increase wear and oil viscosity.
In the U.S., oils intended for use in low-emissions diesel engines must meet the requirements of API CH-4. Deposit control performance of lubricants intended for low-emissions engines is evaluated in the Caterpillar 1P single-cylinder engine test. The Roller-Follower Wear, Mack T-8E and Mack T-9 tests measure a lubricant's ability to guard against serious soot-related problems such as valvetrain wear, viscosity increase and filter plugging.
In addition, lubricants to be used in four-stroke diesel engines operating on high-sulfur fuels must meet the requirements API CF, while lubricants to be used in modern two-stroke diesel engines must meet the requirements API CF-2.
Similar requirements are influencing the development of engine oil requirements in Europe, where ACEA has issued diesel engine oil specifications for both light-duty dieseland commercial vehicles.
U.S. Military
The U.S. military diesel engine oil specification, MIL-PRF-2104G, covers engine oils suitable for lubricating spark and compression ignition internal combustion engines. Engine performance tests used for MIL-PRF-2104G qualification are the Caterpillar 1M-PC and 1N, Mack T-8, Roller Follower Wear and HUEI Oil Aeration Tests.
MIL-PRF-2104G implements API CG-4, CF and CF-2 diesel engine oil requirements. The specification also covers power transmission fluid applications in combat/tactical service by including the transmission test requirements of Allison C-4and Caterpillar TO-4. These requirements cover graphite, paper and bronze friction; friction retention; gear wear testing; and seal compatibility. It should be noted that compliance with the TO-4 test requirements in MIL-PRF-2104G does not constitute full compliance with the Caterpillar specification.
Gear Lubricants
The primary functions of a gear lubricant are the same as those for all lubricants. However, particular emphasis might be placed on friction reduction and heat removal. Contaminant containment, while important, is not as difficult as with crankcase lubricants because no fuel degradation products are present.
The principal types of additives used for gear lubricants are:
The oxidation, rust and foam inhibitors used in gear lubricants are generally of the same type as those used in crankcase lubricants.
Of particular importance are antiwear and extreme pressure additives, which are activated only under specific temperature and pressure conditions and are inactive under other conditions. This property is necessary both to preserve the reagents and to avoid extraneous reactions that might be detrimental to the system. Examples of harmful side effects are excessive wear on gear teeth, ball and roller bearing parts, and other differential components, as well as possible deposits in oil passages and other critical areas.
Combining the necessary additive properties in a single gear lubricant package is not a simple matter. A major stumbling block is the difficulty in reconciling high-speed and high-torque requirements. Some materials that enhance one type of performance can hinder the other. Because of the complexity involved, many full-scale passenger car and truck axle tests must be run, both in the laboratory and on the road. These tests require the support of numerous chemical and physical bench tests to screen out the most promising candidates.
To completely specify a gear lubricant, both the API service designation and SAE viscosity grade are required. Viscosity should be selected based on minimum and maximum service temperatures. Multigrade fluids are normally used, and each viscosity grade has distinct criteria for low and high-temperature performance.
Passenger car rear axle lubricants require score protection as well as thermal and oxidative stability and rust protection. This is provided through the use of sulfur-phosphorus lubricants. Requirements for these lubricants are described in the API GL-5 specification.
The requirements of many equipment builders exceed those of the API specifications. As a result, SAE and ASTM have updated GL categories to reflect present and future needs. This action has promoted the development of gear lubricant categories API MT-1 and proposed PG-2, which are designed to meet the performance requirements of North American heavy-duty or commercial equipment.
API MT-1 designates oils for heavy-duty truck and bus manual transmissions. Its focus is on improved high-temperature cleanliness and stability, oxidation and antiwear control, and compatibility with oil seals and copper alloys.
PG-2 is for heavy-duty truck and bus final drive axles using spiral bevel and hypoid gears. It also includes requirements for improved high-temperature properties and seal compatibility. Tests included in the proposed specification are:
Tests for Proposed Gear Oil Category PG-2
Required Performance |
Recommended Test |
Thermal Stability/Component Cleanliness |
L-60 (Intevep rating procedure) |
Oil Seal Compatibility |
ASTM D 471 |
Copper Compatibility |
ASTM D 130 (3 h at 121°C) |
Gear Surface Fatigue |
Mack Spalling |
API GL-5 Performance |
Current API GL-5 Tests |
Compatibility with existing gear lubricants |
SS&C FED-STD-791 Method D3430 & 3440 |
The U.S. Military recently updated its gear oil specification to MIL-PRF-2105E. This specification combines the requirements of 2104D and API MT-1. MIL-PRF-2105E requires heavy-duty manual transmission field testing in addition to the heavy and light-duty axle testing currently required.
Many automobile manufacturers have proprietary test requirements for their factory-fill gear lubricants. As such, factory-fill oils provide unique performance characteristics that are critical for the satisfactory operation of a particular OEM's drivetrain. Their performance characteristics may include break-in, bearing preload and limited-slip durability. However, API GL-5 lubricants are often recommended for service-fill applications.
The quality of European gear oils is influenced primarily by the requirements of the major commercial vehicle and passenger car manufacturers. API GL-5 and MIL-L-2105D establish the minimum performance level for European commercial vehicles. Equipment builders then impose additional requirements to meet their specific needs for improved surface fatigue, component cleanliness, synchromesh durability and viscometrics.
The majority of passenger cars now use a transaxle drivetrain arrangement, reducing the need for rear axle lubricants. These vehicles are filled for life at the factory. Conventional API GL-4 lubricants are being replaced by more specialized manual transmission fluids. These fluids have excellent thermal stability and carefully tailored frictional characteristics to provide smooth synchronization and good shift quality.
A performance area not addressed by industry specifications is limited slip. Because of hardware differences among the various limited-slip differentials, no standard industry-wide test is available to evaluate a lubricant's ability to prevent chatter in this application. Lubricant requirements, therefore, are based on performance in an individual manufacturer's test rig or vehicle.
Automatic Transmission Fluids
The principal functions of automatic transmission fluids (ATFs) are:
The general types of additives used to enhance these functions are:
|
|
A critical problem in developing an ATF is providing the desired frictional properties for proper clutch pack operation while still providing the other properties. Because of differences in transmission design among the major auto manufacturers, the required frictional properties vary considerably. One design may require a "slippery" fluid with a low coefficient of friction at lock-up to provide a smooth shift without the noise and wear produced by stick/slip. Another might require a higher coefficient of friction to ensure fast clutch plate lock-up that prevents wear due to excessive slippage.
ATFs must also have a sufficiently high viscosity at elevated temperatures to ensure against excessive leakage in hydraulic and control systems. This would result in low hydraulic pressures and degradation of shift characteristics. In addition, too high a viscosity at low temperatures causes reduced fluid flow, which causes reduced fluid efficiency, pump cavitation, extended shift time, possible clutch plate failures, and reduced starting capability.
Modern vehicle and transmission designs place increased stress on the automatic transmission fluid. The drive to improve fuel economy has led to more aerodynamic car designs that permit less airflow around transmission, thereby increasing operating temperatures. This, combined with reduced sump sizes, results in severe thermal stress on the fluid.
Requirements in both the General Motors DEXRON®-III and Ford MERCON® specifications are aimed at avoiding problems caused by high-temperature operation. The 4L60 oxidation test in DEXRON®-III ensures that the fluid keeps transmission parts virtually sludge free. In addition, the specification severely limits total acid number (TAN) increase. Likewise, MERCON® limits maximum viscosity increase in the aluminum beaker oxidation test to 40% to prevent high-temperature oxidation problems.
Automakers are offering longer drivetrain warranties; therefore, increased emphasis has been placed on improved durability and consistent shift quality. Providing the proper friction performance is a vital role for an ATF. The band and plate friction tests, and 4L60 cycling test in DEXRON®-III, and the MERCON® friction durability test ensure that a transmission retains its shift quality throughout the life of a vehicle.
Electronic controls are common in modern transmissions, and the smaller fluid orifices required for these controls challenge an ATF's rheology, especially in cold weather. To ensure proper operation, particularly at start-up, ATFs must have improved low-temperature viscosity. Therefore, both DEXRON®-III and MERCON® require a maximum viscosity of 20,000 cP at -40°C.
Another important property of and ATF is compatibility with elastomer seals. The fluids can affect the tensile strength, elongation, hardness and volume of elastomers. Various immersion tests are generally used to evaluate the compatibility of ATFs with the different seal materials.
Several other fluid properties are important for the proper functioning of an automatic transmission, including lubrication of the moving parts, foam resistance, low volatility, low pour point, and high flash and fire points. Generally, the technology required to provide these properties is not as complicated as that to produce the other properties discussed.
Source: http://www.todaystrucking.com/images/Lubrication_Theory_and_Practice4.doc
Web site to visit: http://www.todaystrucking.com
Author of the text: indicated on the source document of the above text
If you are the author of the text above and you not agree to share your knowledge for teaching, research, scholarship (for fair use as indicated in the United States copyrigh low) please send us an e-mail and we will remove your text quickly. Fair use is a limitation and exception to the exclusive right granted by copyright law to the author of a creative work. In United States copyright law, fair use is a doctrine that permits limited use of copyrighted material without acquiring permission from the rights holders. Examples of fair use include commentary, search engines, criticism, news reporting, research, teaching, library archiving and scholarship. It provides for the legal, unlicensed citation or incorporation of copyrighted material in another author's work under a four-factor balancing test. (source: http://en.wikipedia.org/wiki/Fair_use)
The information of medicine and health contained in the site are of a general nature and purpose which is purely informative and for this reason may not replace in any case, the council of a doctor or a qualified entity legally to the profession.
The texts are the property of their respective authors and we thank them for giving us the opportunity to share for free to students, teachers and users of the Web their texts will used only for illustrative educational and scientific purposes only.
All the information in our site are given for nonprofit educational purposes