Chapter 1: An Introduction
Materials Science for Engineers
Each word in the textbook title is significant.
Materials
Materials are the substances from which things are composed. Their impact on society cannot be underestimated. Civilizations have been designated by the level of their material advancement: The Stone Age, The Copper Age, The Bronze Age, The Iron Age, etc. How would you characterize the current era?
Primitive humans had access to only a limited number of materials that occurred naturally: stone, wood, clay, skins. Then, techniques were discovered to produce (engineer) materials that had superior properties. The earliest example is probably pottery which has been prevalent for thousands of years. Another example is heat-treated metals. Another is alloys. (Alloys are materials made from metals combined with impurities.) Today's engineers have created extremely sophisticated materials for very high-tech and demanding applications.
Science
The word science refers to the fundamental nature of the physical world. The science of materials refers to their structure and their properties.
Engineering
Ask ten engineers what the definition of the word engineering is and you will get several different answers. The most common will contain the idea that engineering is the field that applies basic science to solve society’s problems. One brilliant engineer said that engineering is the “art of compromise.”
In the field of materials, engineering refers to the processing techniques for materials and to the selection of an appropriate material for a specific application.
How does it all relate?
The fundamental science of materials looks at their structure and properties.
Materials engineering is the processing and selection of materials.
These concepts relate to each as follows.
Processing → Structure → Properties → Application
(The arrows can be read as the word “determines”.)
A material’s structure determines its properties which in turn determine the applications for which it can be used. However, with knowledge of the structure and some creativity, the engineer can formulate a process that will change the structure and enhance the properties, thereby enabling it to be used for a sophisticated application.
So from the knowledge of the fundamental science of materials, engineers develop processes (manufacturing techniques) that dramatically alter the natural (or equilibrium) structure of a material and hence its properties to meet specific requirements for an application.
In the last 100 years, knowledge of the basic science of materials has enabled scientists to engineer materials that meet ever more demanding specifications. The cutting edge nanotechnology promises to create new materials that will revolutionize many fields, including energy production, medicine and electronics.
Example:
The elimination of porosity in Alumina (Al2O3) is a good example of how microscopic structure affects properties.
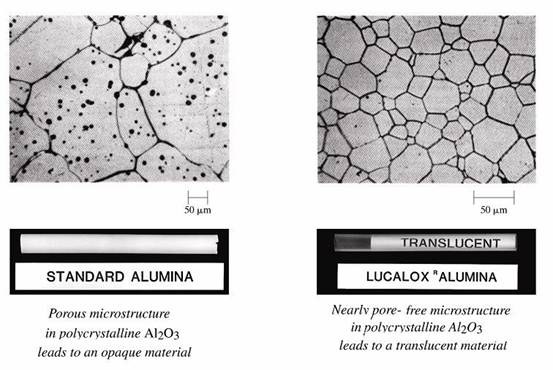
The Structure of Materials
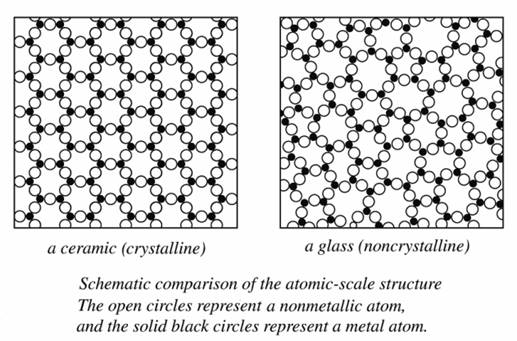
The structure of materials can be discussed on different levels (scales) ranging from the human scale to the atomic scale:
An example of atomic scale structure is whether materials have long-range order (crystalline) or short-range order (amorphous).
Material Properties
Material properties are the inherent characteristics of a material. These can be described qualitatively and quantitatively.
One can qualitatively say that metals are strong and shiny. Or that polymers are ductile. But how strong? How shiny? How much deformation will a polymer experience for a certain load? These kinds of questions relate to the quantification of material properties.
Engineers have quantified and standardized material properties with very careful and very specific definitions. These definitions are published by professional societies such as ASM, ASTM, ASME, ANSI.
For example a material property called Yield Strength is the level of stress (load divided by cross sectional area) at which point the material starts to yield (permanently deform). So this property measures a material's response to a mechanical stimulus. (Usually a material property measures the response to some stimulus.)
Material properties fit into six broad categories bases on the type of stimulus:
1. mechanical properties – measure the response to a load (force)
(e.g. tensile strength, elastic modulus)
2. electrical properties – measure the response to an electric field
(e.g. conductivity)
3. thermal properties – measure the response to heat
(e.g. melting temperature)
4. magnetic properties – measure the response to a magnetic field
(e.g. permeability)
5. optical properties – measure the response to electromagnetic or light radiation
(e.g. index of refraction)
6. deteriorative properties – measure the response to environmental factors including moisture, oxygen, uv radiation.
Classification of Materials
One way to classify materials is in these six categories:
An alternate classification of materials uses categories based on their properties:
Criteria for selection of materials
Engineers must take into account various considerations when selecting materials for an application.
Some of the more obvious criteria:
|
STRUCTURE |
PROPERTIES |
EXAMPLES |
Metals/ Alloys |
|
|
|
Ceramics/ |
|
|
|
Polymers |
|
|
|
Composites |
|
|
|
Semi-conductors |
|
|
|
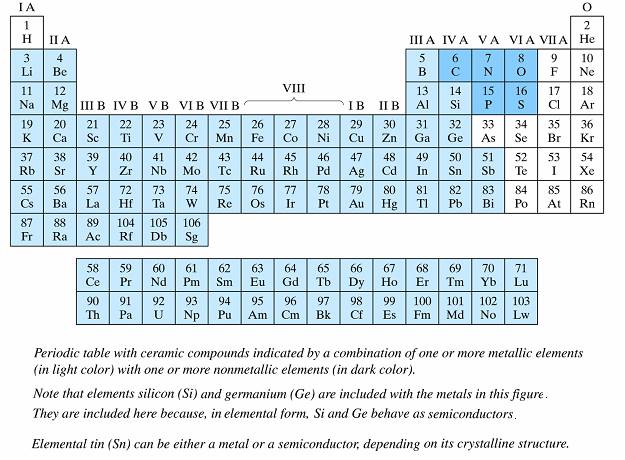
Source: https://fog.ccsf.edu/~wkaufmyn/ENGN45/Course%20Handouts/Chap01_Intro%20Lecture.doc
Web site to visit: https://fog.ccsf.edu
Author of the text: indicated on the source document of the above text
If you are the author of the text above and you not agree to share your knowledge for teaching, research, scholarship (for fair use as indicated in the United States copyrigh low) please send us an e-mail and we will remove your text quickly. Fair use is a limitation and exception to the exclusive right granted by copyright law to the author of a creative work. In United States copyright law, fair use is a doctrine that permits limited use of copyrighted material without acquiring permission from the rights holders. Examples of fair use include commentary, search engines, criticism, news reporting, research, teaching, library archiving and scholarship. It provides for the legal, unlicensed citation or incorporation of copyrighted material in another author's work under a four-factor balancing test. (source: http://en.wikipedia.org/wiki/Fair_use)
The information of medicine and health contained in the site are of a general nature and purpose which is purely informative and for this reason may not replace in any case, the council of a doctor or a qualified entity legally to the profession.
The texts are the property of their respective authors and we thank them for giving us the opportunity to share for free to students, teachers and users of the Web their texts will used only for illustrative educational and scientific purposes only.
All the information in our site are given for nonprofit educational purposes