Glues and adhesives have been made since ancient times and many of the materials were naturally occurring; for example, bitumen and tree resins. The growth of the plastics industry has resulted in the discovery of many new adhesives from synthetic resins.
Many older type glues are still used today but are generally restricted to use on natural materials whereas the modern adhesives will adhere to a wide variety of materials; for example, glass and steel.
In the past most glues or cements have been based on naturally occurring animal and vegetable substances, but recently a range of synthetic adhesives has been developed which give rapid, high strength bonds.
Insufficient time has elapsed to thoroughly test the durability of such adhesives but indications are that the durability is very high in exposed conditions, making these newer adhesives suitable for structural applications.
Properties of adhesives vary considerably with their constituents. For instance:
Different adhesives have:
Adhesives set in a number of ways:
Adhesives derived from starch (like old-fashioned flour-and-water paste), cellulose (eg. methyl cellulose, which is a wallpaper paste), animal by-products (used for wood-wood bonds) and casein (made from soured milk curds and used for wood-plasterboard, wood-linoleum bonds) are only suitable for interior use as they tend to lose their strength when wet and, in the case of animal glues, are susceptible to attack by micro-organisms even with the addition of fungicides.
The one exception is bituminous adhesives which are based on bitumen or coal tar. This group has good resistance to water and many chemicals but they do tend to flow at high temperatures. They are used for laying various flooring materials, such as parquet blocks and vinyl and linoleum sheets and tiles, and for bonding roofing felt.
These adhesives can be based either on natural or synthetic rubber. In general, they are not suitable for external application but have the advantage of a degree of flexibility which can accommodate slight movements between the glued surfaces. This can be useful when bonding wall boards.
They may be used as a one-part system or as a two-part ‘contact’ adhesive-where both surfaces are coated and then brought together to achieve an instant bond after enough time has elapsed for the solvent to evaporate. Contact adhesives are very suitable for bonding plastic laminates and sheet floor coverings but there is no margin for error-you must get it right the first time.
They are not generally suited to wood joints as the adhesive tends to flow under constant load.
These adhesives fall into two groups, those based on polyvinyl acetate (PVA) and those which are described as ‘hot-melt adhesives’.
Used mainly for wood working but suitable for a wide range of materials, these adhesives are white liquids which become transparent on setting and generally do not discolour materials, except in some cases in the presence of ferrous metals.
PVA’s are easy to use, they set at room temperature and do not blunt cutting tools.
PVA is generally suitable for joints which will not be required to undergo high continuous stress.
Usually available as a single-part system, PVA is slightly more waterproof than animal glues but is restricted to interior applications, nevertheless.
As the name suggests these adhesives are usually applied in a hot molten state.
They are suitable for continuous flow production, are not flammable and the bond is formed in seconds.
Sealing wax is an example of this type of adhesive, but modern varieties are usually based on ethylene vinylacetate (EVA) copolymers.
Capable of extremely high strengths, even for metal-metal bonds, these adhesives harden essentially by heat action in conjunction with a catalyst or hardener which allows reasonable curing times at room temperature.
Disadvantages are that they are combustible and require special cutting tools.
They are available either as a one-part or two-part system.
These adhesives are colourless and inexpensive and are widely used in building but are unsuitable for external applications.
These adhesives are not affected by weather or boiling and are therefore suitable for manufacturing marine ply.
Relatively expensive and colourless, these adhesives are suitable for work such as veneering where increased durability and heat resistance is required.
Some of the adhesives in this group have remarkable properties which tend to offset their high cost.
They also have the advantage of setting at room temperatures.
This adhesive can be used at low temperatures and, although it is water soluble until cured, when hardened it is weatherproof and boilproof.
It is used for extremely strong and durable joints in timber and is also suitable for plastics, and alkaline materials such as fibrous cement sheets.
Although expensive, these two-part adhesives (eg. ‘Araldite’) will bond almost any materials.
In addition, they are waterproof, resistant to most chemicals, highly electrically resistant and very resilient.
Shrinkage is negligible during curing.
As they are transparent, they are suitable for frameless glass assemblies, such as show cases.
These are costly, one-part adhesives which form an instant bond (eg. ‘Superglue’).
The bond produced is extremely strong but the glue tends to fill gaps between the two surfaces poorly.
Instant adhesion to skin can present a serious hazard.
Adhesion may be due to molecular attraction between two surfaces (as occurs between two sheets of damp glass), or to bonding agents which key into porous surfaces, or both. Modern adhesives work in both ways.
For maximum bond strength it is important not to use too much adhesive so that the surfaces are brought into close contact with a thin glue line.
Contact adhesives give instant tack but, generally, surfaces must be clamped together (but not too tightly) until a bond is achieved.
Surfaces to be bonded must be clean, dry and free from grease.
In some cases they need to be roughened or etched.
A mastic is a sealant which usually provides little structural support but seals the joint against weather and sound while allowing the different components to move relative to each other.
The most common mastic used in domestic construction is linseed oil putty for glazing timber sashes but modern mastics are now available which can be either of the plastic or elastic type.
Plastic mastics are often called sealants and are more expensive and more durable than the elastic mastics. They usually remain plastic for a period of time before hardening to a point where loads can be sustained without squeezing out.
Elastic mastics can be based on silicone, polyurethane, butyl rubber or polysulphide rubber. They are used to seal a variety of assemblies including glazing and metal curtain-walling, around baths, sinks and basins and joins in wall tiling.
As constituents vary considerably, manufacturers’ recommendations should be studied carefully. Points to consider when selecting a mastic include resistance to moisture penetration, exposure to weather, exposure to chemicals, compatibility with adjacent materials, loading conditions and ease of application.
Source: http://bctcwagga.riverinainstitute.wikispaces.net/file/view/Unit+8+Plastics+and+Adhesives.doc
Web site to visit: http://bctcwagga.riverinainstitute.wikispaces.net
Author of the text: indicated on the source document of the above text
Overview of Mixing Technologies
For the Production of Low to High Viscosity Adhesive Applications
The preparation of almost all adhesives begins and ends with adequate mixing. From the homogenization of adhesive emulsions, to the dissolution of polymers into solvents, or mastication of rubber and let-down of master batches, the type of mixing equipment and method hugely dictate over-all processing efficiency and end-product quality. This article seeks to provide an overview of effective and updated mixing technologies being implemented across many of today’s competitive adhesives manufacturing plants, as well as new equipment designs increasingly being recognized by the industry as potential solutions to prevailing mixing challenges.
HIGH SHEAR MIXERS
Early equipment used to dissolve polymers into solvent was based on low speed propeller, turbine or rake type agitators in vessels (known as churns). These devices relied heavily on the solvent’s softening action on the polymers and predictably yielded very long cycle times. Mixing in a churn for as long 12 – 24 hours was typical. The operator would load the vessel with raw materials, turn on the mixer in the morning and shut it off in the evening or the next day. This problem was exacerbated when the resin was supplied in pellet or slab form, making it difficult to dissolve. Even with the introduction of saw-tooth type high-speed dispersers, batch times could take up to several hours just to dissolve the resin.
To hasten the solvation process, a high shear mixer is recommended. Composed of a four-blade rotor that turns at high speeds within a stationary stator, a high shear mixer will mechanically shear large particles and reduce their size. Materials are drawn from below the mixing head and expelled at high velocity through the openings of the stator creating intense hydraulic and mechanical shear. As fast as material is expelled, more is drawn into the rotor/stator generator. Polymer particles are thus broken down into smaller and smaller pieces which get easier and easier to dissolve. Fillers too such as fumed silica are dispersed faster with a high shear mixer compared to lower energy devices.
To illustrate, a manufacturer of PVC solvent cements switched to the Ross rotor/stator mixer from a high speed disperser and reduced their cycle time from four hours to just under 45 minutes when PVC pellets are used, and under 30 minutes when the PVC is in powder form.
Another company making insulation adhesives experienced the same >80% reduction in mixing time when they replaced their disperser with a Ross high shear mixer. The batch rotor/stator efficiently tears up pieces of neoprene rubber and assists in a more rapid dissolution into a blend of toluene, hexane and acetone solvents. The use of a closed and jacketed mix vessel prevents the loss of solvents and also allows the operator to control batch temperature.
The high shear mixer, available in both batch and inline configurations, is not only useful for dissolving solids into liquids, but also for preparing emulsions. A manufacturer of protective films and tapes used for a variety of surfaces including carpets, windows, marble and steel was looking for a faster way of blending two liquid components of an adhesive emulsion. They were using a propeller type mixer which they had to run for one hour to ensure a homogenous low-viscosity mixture made of 95% acrylic emulsion and 5% polyisocyanate solution. Target droplet size was 0.30 microns or below. Laboratory tests revealed that an inline high shear rotor/stator mixer was able to achieve the desired product characteristics in just a single pass. This translated into a dramatic improvement in mixing time using a relatively small inline mixer.
In a good number of cases, due to the drastic reduction in mixing time, shifting from a low-speed, low-shear mixing system to a high shear rotor/stator generates significant savings in power consumption, which results in a full return of investment within a very short period of time.
SOLIDS INDUCTION SYSTEMS
Ross inline high shear mixers with Solids/Liquid Injection Manifolds (SLIM) are utilized in continuous or recirculation operations where significant amounts of solids must be added into a low-viscosity liquid. The SLIM consists of a special rotor/stator arrangement designed to create negative pressure behind the rotor, which acts as a motive force to suck powdered (or liquid) ingredients directly into the high shear zone where they are instantaneously mixed with the incoming liquid stream. The resultant mixture is then expelled centrifugally through the openings in the fixed stator before exiting the discharge connection.

Yellow stream: Incoming powders
Blue stream: Incoming liquids
Green stream: Outgoing Mixture
For years, a developer of automotive adhesives and coatings had been using a high-speed dispersion mill to prepare a high volume adhesive premix. Crumbs of chlorinated rubber, different grades of carbon black and other fillers were dispersed into a solvent solution. Although adequate in attaining the desired grind prior to the sandmilling step, the dispersion mill was prone to overheating and required a great deal of maintenance. Looking for a better way to prepare their premixes, this company tried the Ross inline SLIM at their facility. Extensive trials confirmed that the SLIM system offered a three-fold advantage to their dispersion mill: lower processing temperature (85oF vs. 140oF), higher solids loading capability (28-33% vs. 8-12%), and faster, dust-less powder induction.
In a SLIM system, powders can be sucked from the bag using a flexible wand connected to the solids inlet port or powders may be transferred into a hopper right above the mixing chamber. Still another method of transporting powders to the SLIM is via an automated feeding device such as a bulk bag and feeder combination. Use of the SLIM system greatly reduces dusting.
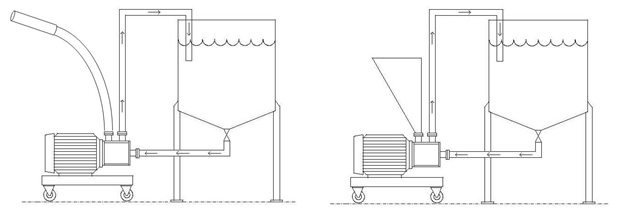 Flexible Hose & Wand Attachment Hopper Attachment
Flexible Hose & Wand Attachment Hopper Attachment
A producer of pressure sensitive adhesives was using a steam-jacketed mix tank with a center agitator blade and a counter rotating sweep blade to disperse rosin ester resin powders into a surfactant-water solution. This process took 5 to 6 hours to complete. Temperature was closely monitored and kept below 113oF. At higher temperatures, the resin particles would soften and begin to agglomerate into sticky clumps, which affected product quality and made cleaning difficult. When this point was reached, cooling was necessary to reverse the agglomeration.
Evaluation of a 25HP inline SLIM system with a hopper attachment revealed that resin powders can be inducted under high shear at an impressive rate of around 75lbs/minute. Due to the very short cycle time, the 45% resin mixture is kept well within the 113oF limit and a cooling step is not required.
The SLIM system is also available in a batch design. Other applications of the SLIM technology include the induction of fumed silica, bentonite clay, calcium carbonate, cellulose gum, CMC, flour, starch and talc powders.
ULTRA-HIGH SHEAR MIXERS
In some instances, even conventional four-bladed rotor/stator mixers are unable to provide the required level of shear for dispersion, emulsification or particle size reduction. In these cases, manufacturers commonly turn to high-pressure homogenizers or colloid mills to make their adhesive products.
Yet, several issues are typically encountered which make the use of homogenizers and mills less than ideal. These issues include low throughput, labor-intensive and time-consuming clean-up procedures, and high maintenance (i.e. life of seals are relatively short due to the extreme operating conditions). In addition, high-pressure homogenizers have a high comparable initial cost.
A different and welcome approach comes in the form of new generation ultra-high shear mixers, an emerging technology being recognized across a wide range of process industries and not just in the field of adhesives.
Ultra-high shear mixers have unique rotor/stator geometries and run at tip speeds as high as 18,000fpm. The combination of extremely close tolerances, specially designed channels and grooves in the rotor/stator generator and high tip speed, not only produces sub-micron dispersions and emulsions, but also disintegrates solid polymers into extremely small particles in just a single pass.
The Ross MegaShear, X-Series and QuadSlot Ultra-High Shear Mixers represent a quantum leap over the conventional rotor/stator mixer technology. Speeds, rotor/stator gap settings and residence time can be controlled to prevent over-shearing, which can produce a broken emulsion or degrade the polymer.
In the manufacture of adhesive emulsions, ultra-high shear mixers, compared to lower energy devices, produce finer droplet sizes and may allow the use of less surfactant, which in turn can reduce the cost of the end product. One problem associated with wetting agents and defoamers is that they can be adsorbed by the polymer particles, thereby altering the coating performance of the emulsion. This often leads to product rework and/or scrap. Thus by using less surfactants and defoamers, one can save in raw material costs and also produce more consistent batches. Ultra-high shear mixers present adhesive manufacturers this option.
MULTI-SHAFT MIXERS
Generally, the bulk of the solid portion of an adhesive is the bonding agent and the solvent is only a carrier to provide an easy method of application. Therefore, adhesives with a higher solids percentage usually contain more usable adhesive per gallon. Many adhesive slurries, cements and pastes are high-solids, viscous formulations that cannot be processed in a single disperser or rotor/stator mixer. This is when multi-shaft mixers are employed.
Multi-shaft mixers are comprised of two or more independently driven agitators working in tandem. A low-speed anchor compliments one or two stationary high shear devices (a high speed saw-tooth disperser blade or a high shear rotor/stator mixer). On its own, a disperser blade will produce acceptable flow patterns for products around 50,000 cps maximum; the rotor/stator’s recommended viscosity limit is even lower, around 10,000 cps. Hence, there is a need for a supplementary agitator to improve bulk flow, deliver viscous product to the high shear devices and constantly remove product from the vessel walls for better heat transfer.
The most common low-speed agitator designs are the two-wing and three-wing anchors. For added efficiency, especially in terms of axial flow, the three-wing anchor can be modified to feature helical flights in between the wings, or the vertical wings can be entirely replaced with helical ribbons supported from the top and bottom. Multi-shaft mixers typically process adhesive formulations as high as 500,000 cps.
Aside from the extended capability of multi-shaft mixers from a viscosity standpoint, another design advantage is that they are closed systems that offer the benefit of optional vacuum or pressure mixing.
When processed under vacuum, certain adhesives and composites develop higher densities and possess better tensile properties as a result of improved shearing and contact of the different components. With other adhesive products, vacuum mixing keeps entrapped oxygen to the minimum, which ensures longer shelf life and improved stability (nitrogen blanketing is another technique). Mixing under vacuum also gets rid of unwanted voids that agitation under atmospheric conditions can produce. Pulling vacuum while mixing can also eliminate costly downstream de-aeration steps and shaves overall processing time.
It is common for multi-shaft mixers to function as specialized reactors equipped with automated controls and PLC-based recipe systems. For example, in a polymerization reaction, the batch has to be kept constantly homogenous and carefully monitored for temperature, level, pressure, etc. The properties of the end product are directly influenced not just by the purity of raw materials and reaction chemistry but also by the efficiency of mechanical mixing. Undoubtedly, identical formulations produced in different mixing systems can result in dissimilar stability, adhesion performance, and heat resistance.
PLANETARY MIXERS
Adhesives processed in highly viscous states can easily surpass the limits of multi-shaft mixers. In a multi-shaft mixer, the low-speed anchor can reach a point where it can no longer produce adequate flow – it simply carves a path throughout the batch instead of pushing product away from the walls and into the center. High-temperature zones right near the disperser and rotor/stator assemblies will begin to form. At this point, stationary agitators no longer suffice and a move to a planetary mixer is required.
The agitators of a planetary mixer rotate and travel throughout the mix vessel, passing every point within the batch, not just along the periphery. Highly viscous materials are literally carried from the vessel wall to the batch interior. In essence, a planetary mixer provides powerful kneading and mixing action regardless of the product’s flow characteristics.
The double planetary mixer is almost always a great choice. It can be equipped with traditional rectangular stirrer blades, finger blades or high viscosity “HV” blades. The latter is a patented blade design of Charles Ross & Son Company, which generates a down-thrust action due to its precisely angled helical contour. The sweeping curve of the HV blades firmly pushes material forward and downward, a unique mixing action that solves the ‘climbing’ problem commonly experienced when processing highly filled adhesives. In addition, the HV blades do not have a lower crossbar so they can be lifted cleanly off a very viscous batch and also pierce right through one just as easily.
The order of raw material addition in a double planetary mixer is a crucial parameter. One method is to start with all or majority of the solids (resins, fillers, antioxidants, curing agents and other additives) and gradually add liquids (oils and tackifiers). Unless there are waxes or resins that need to be melted, it is recommended to artificially raise the viscosity by withholding some of the liquids. The higher the product viscosity during mixing, the greater the shear that the planetary blades can impart.
There are adhesive products that require a two-step approach to assure proper dispersion. For instance, after blending all ingredients in a double planetary mixer, the entire batch is transferred to a single shaft, high-speed disperser to provide the extra shear needed for completion. This cumbersome, two-step process is highly labor intensive and time consuming. To improve production efficiency, manufacturers can utilize a hybrid planetary mixer that combines the traditional thorough mixing action of a planetary mixer with the added benefit of a high-speed disperser. Both the planetary blade and the high-speed disperser rotate on their own axes while revolving around a central axis. The planetary blade orbits through the mix can in a circular manner, continuously sweeping the vessel walls, as well as the vessel bottom, and carrying material toward the high-speed disperser. The planetary blade also insures that any heat created by the disperser is evenly distributed throughout the mix. Variable speed allows precise control of shear rates to minimize the degradation of any shear-sensitive components.
Like multi-shaft mixers, double planetary and hybrid planetary disperser mixers used in the production of adhesives also benefit from vacuum processing capabilities for the same reasons: to minimize exposure to oxygen, remove unwanted voids and improve dispersion. In this aspect, vacuum-rated planetary mixers designed for high viscosity processing are more advantageous than traditionally accepted horizontal sigma blade mixers.
Kneader extruders and conventional double arm sigma blade mixers are high torque machines that can muscle through hard blocks of rubber. They remain to be the most powerful tools for manufacturing extremely viscous adhesive formulations. However, there are several considerations, which make planetary mixers a better choice for certain applications.
One of those considerations is that a horizontal mixer can be too powerful and degrade shear-sensitive components such as fibers. Also, this type of mixer relies on the product being highly viscous at all times in order to mix properly – liquid components must be added very slowly, portion by portion, or else they can act like a lubricant and reduce shearing efficiency. A vertical double planetary mixer is more flexible in this regard.
Other comparisons are explained below:
A vertical mixer design has no shaft seals, bearings, packing glands or stuffing boxes submerged in the product zone. In addition, the agitators are raised and lowered in/out of the mix can by a hydraulic lift. This allows easy access for cleaning between batches. Mix cans are interchangeable for designation to a particular formulation and/or color. There is no concern for cross contamination from batch to batch.
Interchangeable blade designs include:
1. Rectangular Blades – Proven choice for many low to high viscosity mixing applications. Provides a powerful kneading action suitable for thorough wet or dry blending regardless of the product’s flow characteristics.
2. Finger Blades – Often preferred for special applications that require the mixing of delicate fibers and solids. This design may also be fine-tuned for custom axial flow mixing requirements.
3. HV (High Viscosity) Blades – PatentedRoss design used for ultra-high viscosity mixing applications. Prevents “material climb” up the blades and has the ability to mix formulations up to 8 million cps.
Footprint of the double planetary mixer is considerably less than that of a double arm / sigma blade mixer.
Since the double planetary mixer uses less motor horsepower to operate, everyday energy/operating costs will be less. This can be significant over time.
With the use of extra mix cans, the double planetary mixer can produce material in a semi-continuous basis: one can is being charged while other cans in the loop are being mixed, QC’d, discharged, and cleaned.
Variable speed is normally achieved through the use of a variable frequency drive (inverter), not an expensive-to-maintain mechanical vari-drive/belt box.
Normal wear items (gaskets, O-rings, packing, etc.) are available from stock.
Depending on specifications, a double planetary mixer is generally 1/2 – 1/3 the cost of a comparably sized new sigma blade / double arm mixer.
VALUE OF TESTING
A manufacturer of corrosion-resistant, trowellable adhesives had developed a new product line of two-component adhesives. At that time, six different mixes were being produced in laboratory scale and the production plant intended for the new line was just being built. A large order came in and engineers had to scramble to get the production equipment.
The adhesive products were relatively viscous (700,000 to 1,500,000 cps) and difficult to handle. Attempts to mix it properly in the plant’s existing sigma blade mixer were not successful – the product had too many voids and there was evidence that the reinforcing fibers were being degraded.
A test run in the Ross Test & Development Center confirmed that a double planetary mixer was appropriate for the application. The adhesive company rented a production-scale unit for further evaluation at their plant. Burnout tests and visual examination showed that the planetary mixing action did not degrade the reinforcing fibers in the mixture. The resulting product possessed the required high strength and exhibited maximum tensile properties.
The mixer performed very well and the plant decided to keep it, as well as purchase two extra mix cans to accommodate the mixing of multiple components and multiple batches. Once a mix is finished in one vessel, it is taken to the packaging area for emptying while another mix can is positioned under the double planetary mixer. During production, the plant tries to reserve a can for each recipe. As long as the components are not being changed or left in the vessel longer than specifications permit, clean up can be avoided. Lifting the mixing head and wiping them with the appropriate solvent easily clean the planetary blades.
CONCLUSION:
The success of modern adhesive chemistries can only go so far as our capability to produce them cost-effectively. It is only essential for process engineers to be updated on the different mixing technologies that are being improved or made available. Many of these mixers’ uses and functions overlap such that two or more types of mixing systems can actually successfully produce certain applications. For example, many types of epoxy-based adhesives, contact cements, hot-melts and pressure-sensitive adhesives can be made in heavy-duty multi-shaft, double planetary and hybrid planetary mixers. In these cases, economics rules out the more costly initial investments but must also take into account the difference in efficiencies, i.e. how much faster is one mixer able to make a batch compared to another system or how much less power is required?
A visit to a mixer manufacturer’s test center will be very helpful and will make your selection process easier. Be sure to test a variety of equipment and techniques using your own raw materials, simulating conditions as close to your actual process as possible. Quantitative test results provide the best assurance that you have chosen the best mixing system for your particular adhesive product.
Source: https://www.mixers.com/whitepapers/overview%20of%20adhesives%20mixing%20technologies.doc
Web site to visit: https://www.mixers.com
Author of the text: indicated on the source document of the above text
Andrew Clements
This paper presents an overview of the issues involved with selection of an optomechanical adhesive. It proposes a very general approach by which selection of optomechanical adhesives may be considered. It gives a brief historical overview of the major developments in the field of optical adhesives. It discusses many of the important material properties that are used to characterize and distinguish optomechanical adhesives. It presents common ways in which adhesives are categorized. It mentions some applications that place more stringent requirements than usual upon adhesives and the challenges they present. It discusses the failure mechanisms of adhesives and some of the things that can go wrong in the processing of adhesives. Finally, it addresses some issues that may be pertinent to applications that demand adhesives with good chemical and solvent resistance.
Adhesives are very useful materials with widespread application in optomechanics. At the most basic level, the purpose of an adhesive is to join two parts together. There is a host of reasons for using adhesives rather than mechanical fasteners in different applications. For example, one or both of the materials may be brittle and prone to fracture if threaded. The application may require fixing the pieces together without obscuring the transmitted optical path, thus demanding an optically transparent adhesive. The application may take advantage of the ability of adhesives to more broadly distribute stresses than mechanical fasteners. Adhesives also have their drawbacks, prime among which are the host of thermal issues that they introduce.
When an application has been identified which could benefit from use of an adhesive, the task of the engineer is to determine which adhesive to choose from among the plethora which are now available. Unfortunately, there is no magic formula that one can use to quantitatively determine the best adhesive for the particular application. Likewise, there is no one adhesive that is suitable to all applications. Selection of adhesives becomes a balancing act of trading off several material properties according to the priorities of the particular application. This can be further complicated by the fact that the properties of adhesives are dependent upon their processing and curing parameters as well as their use environment.
While making no pretense of presenting an all-purpose formula or procedure for selecting optomechanical adhesives, this paper will discuss many of the issues pertinent to identifying candidate adhesives that are likely to be well suited to an intended application.
If any approach at all could be suggested, I would recommend first becoming familiar with the material properties that characterize and distinguish optomechanical adhesives, to understand which of those properties are the most important for the intended application, and to try to narrow the search down to one or two classes of adhesives that would best address the main requirements of the bonding application. From there, one would seek out suppliers of this type of adhesive, survey their products, and choose some formulations as prime candidates based on a review of online selection tools and telephone technical support. After a second look to determine if any details about the material would disqualify it for the application, one should request samples and test a handful of the most likely materials, trading off performance, cost, and secondary material properties. With the large array of suppliers and the wide variety of adhesives that each supplier offers, finding the optimal product for a particular application may require a bit of work and a fair amount of luck, but finding an acceptable adhesive in fairly short order.
When selecting optical adhesives, it is useful to have at least a cursory understanding of the historical development of optical adhesives so that the extensive offerings available today can be put in some perspective. The following is a brief summary of the history of optical adhesives, as set forth in a very informative paper by James T. Magyar. The reader is directed there for additional detail.
The original optical adhesive was Canada Balsam, the distilled and filtered sap from the balsam tree. It had limitations at high and low temperatures, which became apparent during its use in optical instruments in World War II. A replacement, dubbed M1-10-2A, was developed in 1945-1946 that withstood a temperature range of –50 to +100 degrees C. However, it required a cure temperature of 100 degrees C. Since the bond was achieved at such an elevated temperature, the stresses that resulted from returning to room temperature often cracked the optical elements. In 1959, it was reformulated to include a cobalt catalyst, which allowed curing in 4 days at room temperature or in 2 hours at 70 degrees C. This became the industry standard MIL-A-3920 and is sold today by Eastman Kodak and Summers Laboratories under the designations HE79 and HE80 (Kodak) and Lens Bond Type C-59 and Type M-62 (Summers). High production volume and demanding tolerances began to demand an optical cement that could pre-cure in seconds rather than minutes and full-cure in minutes rather than hours. Summers introduced the first UV-curing adhesive in 1966 to meet this need.
Since then, the number of available optical adhesive formulations has skyrocketed. Paralleling this has been further development in structural adhesives suitable for optomechanical applications. The selection available today is considerable.
To make an informed selection of optomechanical adhesives, it is important to be well versed in the material properties which distinguish one formulation from another, and to understand which of these properties are most important for the intended application. This section presents a brief discussion of some of the commonly reported material properties, including properties which characterize the adhesives’ ease of use before cure, cure processing, and optical, mechanical, thermal, and chemical properties after cure.
3.1. Pre-Cure Properties
The shelf life of an adhesive (how long it can be stored without a significant change to its properties) may or may not be an important factor in the selection of an adhesive for a particular application, but it is important to know for the proper use of adhesives. A typical shelf life for two-part epoxies is around 6 to 12 months. One-part epoxies are typically much shorter than this. Manufacturers may recommend ways to extend shelf life such as refrigeration or freezing.
This applies to 2-part systems and is the time during which an adhesive can be used after mixing without a significant change in viscosity. It is usually defined as the time from mixing to a 10% increase in viscosity. This can vary widely, from 30 seconds to 5 days, and can be an important consideration in the selection of an adhesive, depending on the requirements of the fabrication process. Adhesives with longer pot life are easier to work with, but may result in longer full cure duration than might be desired for quick fabrication of test samples or high volume production.
Viscosity is defined as resistance to flow or shear stress. It is an important property in determining the ease of handling of an adhesive in its uncured state. It is easy to control the bead size and position of high viscosity adhesives. Low viscosity adhesives tend to have improved wetting. However, very low viscosity adhesives will run and are consequently hard to control. Viscosity is directly proportional to temperature. It is important to consider this, since an adhesive that has a good viscosity for room temperature application may flow under a high temperature cure. Viscosity is measured in centipoise. Optical adhesives can vary widely in viscosity from about 100 cps for a low viscosity UV-cured acrylic to about 90,000 cps for a two-part silicone.
Wetting is the ability of an uncured adhesive to make intimate contact with the substrate in order to facilitate a bond. The important physical parameter related to this is surface tension. An adhesive must have a lower surface tension than the substrate to make good contact and avoid beading. As a rule of thumb, the surface tension should be about 10 dynes/cm below the surface energy of the substrate. Most optical adhesives have a surface tension of around 30 to 35 dynes/cm.3
The details of the cure process for the adhesive can be important factors in its selection. Adhesives can cure in as little as 30 seconds or may require several days to achieve a full cure. Adhesives may be curable at room temperature, may require an elevated temperature for curing, or may have a range of cure schedules that offer some compromise of cure time and temperature. Some curing processes are photosensitive and require a certain type of UV light source. Others are moisture sensitive and may require a particular level of humidity for curing. Note that the final properties of the cured adhesive often depend upon the details of the cure temperature and schedule. Further details are discussed below in the section on processing methods.
Out-gassing is the liberation of volatile constituents by the adhesive. This can occur in the uncured state, during cure, and throughout its lifetime after cure. It can occur at room temperature, but is particularly likely upon exposure to vacuum or elevated temperature. Out-gassing is important for two main reasons. First, it can result in shrinkage as the adhesive volume decreases due to the lost gases, resulting in stress. Also, the liberated volatiles can thereafter condense on nearby cooler surfaces and potentially degrade optical surfaces or attack structural or electrical components. In addition, out-gassing can be critical in high-power laser applications because it can result in laser-induced optical damage. Out-gassing is quantified by two measurements: TML (Total Mass Lost, in %) and CVCM (Collected Volatile Condensable Material, in %).3 Out-gassing is most critical in space applications. NASA maintains an excellent guide for selecting low-outgassing materials compatible with spaceflight. In general, the higher the cure temperature, the less outgassing the material will experience after cure.2 Using solvent-free (100 percent solids) adhesives will prevent voids due to solvent evaporation upon cure. However, some small bubbles can still result from dissolved air or water in the adhesive. To prevent this, the adhesive should be de-gassed before use. If the adhesive is 2-part, it is mixed and then placed in a vacuum to remove any trapped air.2
All adhesives shrink in volume at least somewhat during cure. Older solvent-loss adhesives were particularly notorious for this. Modern epoxies typically have about 3-5% shrinkage upon cure. UV-cured adhesives can be as low as 0.2%.3 Modern solvent-free (100 percent solids) formulations minimize shrinkage because no solvent is released during the curing process. Low shrinkage is necessary both to minimize stress and to provide long term stability.
3.3 Optical Properties
Spectral transmittance is an important consideration for optical adhesives. Figure 1 shows the spectral transmittance of a representative optical cement, Norland Products’ NOA 61. The two primary issues of concern are usually coloration and attenuation. Ideally, an optical cement should have a very flat spectral transmittance curve throughout the spectrum of interest, thus imparting no coloration to the scene. Likewise, the transmittance should be high in the spectral region of interest. Spectral transmittance plots are usually given for some specified bond thickness. Transmittance (or attenuation) at some other bond thickness can be calculated according to Beer’s law:
t = e-ax,
Where t is the transmittance at some wavelength, x is the bond thickness, and a is the attenuation constant.
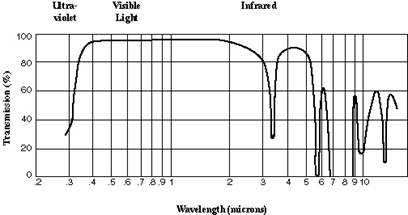
Figure 1. Spectral transmittance of Norland NOA 61 adhesive.
The refractive index of an adhesive is also important for optical cements. The closer the refractive index can be matched to the substrates, the lower the Fresnel reflection losses will be.
Properties describing the elastic behavior of adhesives include Young’s modulus (E), the shear modulus (G), Poisson’s ratio (n), and the bulk modulus (B). Given any two of these properties, the other two can be derived from the relations:
and
B = E / [3.(1-2n)]
3.4 Mechanical Properties
3.4.1 Modulus of Elasticity (Young’s Modulus)
Normal, or longitudinal, stress (s) is defined as the applied force divided by the cross sectional area. (See fig. 2.) Longitudinal strain (e) is defined as the elongation from an applied force divided by the original length. (See fig. 3.) For small deflections of homogeneous material, stress is linearly proportional to strain. The constant of proportionality relating the two is Young’s modulus (E), according to the relation:
s = Ee
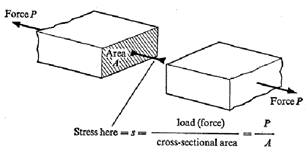
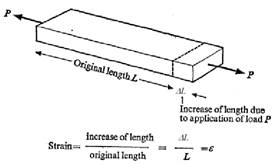
Figure 2. Longitudinal stress. Figure 3. Longitudinal strain.8
Materials with a high Young’s modulus are rigid, whereas materials with a low Young’s modulus are compliant. Adhesives usually have a much lower Young’s modulus than the materials that they are bonding. Thus, the strains experienced by the adhesives are typically much larger than those experienced by the substrates.7 Conversely, if an adhesive has a high Young’s modulus, it will experience high stresses as a result of shrinkage upon cure or differential expansion due to mismatch in thermal expansion coefficients, which may approach the strength of the adhesive.3 An adhesive with a low Young’s modulus can provide stress relief as the material stretches. The challenge with adhesives is to strike a balance between being compliant enough to accommodate stresses due to differential expansion, vibration, etc., yet being rigid enough to resist sag or shifting.5
When a material is in axial tension, there will generally be a strain in the transverse direction as well, opposite in sign to the longitudinal strain (see fig. 4):
ex = Dw / w
Poisson’s ratio is the ratio of transverse strain to longitudinal strain:
n = -ex / ez
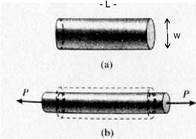
Figure 4. Transverse and longitudinal strain.8
Poisson’s ratio is by definition limited to values between 0 and 0.5. Some substances approach these limits. Cork has a Poisson ratio of nearly zero and rubber has a Poisson ratio of nearly 0.5. Most materials have a Poisson ratio between 0.25 and 0.35. A Poisson ratio of 0.5 means that the material maintains constant volume when it is deformed.
Adhesives with high Poisson ratios behave much differently than their Young’s moduli would predict when they are constrained into thin layers. When a thin bond is put into compression, the thickness of the adhesive layer is slightly reduced. If it has a high Poisson ratio, the adhesive will try to maintain constant volume, but the only place for the material to go is to bulge out at the free edges. In thin bonds, not much free surface area is exposed so not much material can be displaced, and the material exhibits a stiffening that exceeds the Young’s modulus. This new “apparent modulus” behaves exactly like the Young’s modulus (i.e. the ratio of stress/strain) but is a function of the bond thickness. More precisely, it is a function of the ratio of the thickness of the bond over the bond diameter. For thick bonds (i.e. thickness larger than diameter), the apparent modulus approaches Young’s modulus. For very thin bonds (i.e. thickness less than 1/100th of the bond diameter), the apparent modulus can approach the bulk modulus. (See fig. 5.)
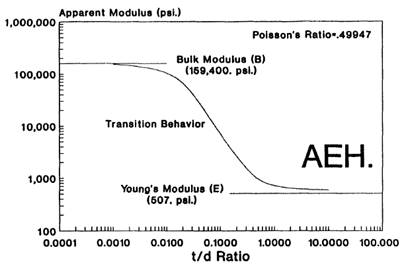
Figure 5. The apparent stiffening of an elastomer in thin bonds.7
Shear stress (t) is defined as shear force (V) distributed over area (A). (See fig. 6.) Shear strain (g) is the angular deformation (in radians) caused by the shear stress. (See fig. 7.) The constant of proportionality that relates these two quantities for homogeneous materials and small deflections is the shear modulus (G), according to:
t = Gg
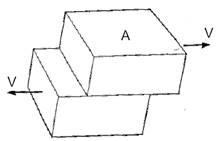
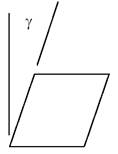
According to the above relations, the shear modulus is necessarily limited between values of:
Note that the shear properties of an adhesive are unchanged by compressing it into a thin layer.7
The bulk modulus (B) is a measure of the compressibility of a material. If a material is subjected to a hydrostatic pressure, P, it will have a corresponding change in volume:
DV/V = ex + ey + ez
The bulk modulus (B) is defined as:
Hardness is defined as the resistance to indentation (after full cure). Hardness is usually characterized by a “Shore D” value. A wide range of hardness values is possible among the various adhesive types, from around Shore D 25 for a soft silicone elastomer to a Shore D 87 for a polishable fiber optic epoxy (e.g. EPO-TEK 353ND).
The strength of an adhesive indicates the maximum amount of stress that can be applied in a particular geometry (i.e. tensile, compressive, shear, peel, or cleavage) without failure of the adhesive. For permanent bonds, this should be high; for temporary bonds, it should be low. Structural adhesives typically have a tensile or shear strength in excess of 1,000 psi. Lapshear strengths are most commonly reported.3
This indicates how far the adhesive can be stretched before it fails. Note that much of this deformation is plastic, so this cannot be easily calculated using the Young’s modulus of the adhesive. This is an indicator of how much differential expansion an adhesive can withstand.
This is the name of the game with adhesives and is obviously a very important property. Good adhesion is absolutely necessary to withstand shock, temperature cycling, and differential expansion. But, it can be tricky to quantify. Adhesive suppliers usually describe the general types of materials (e.g. glass, metal, plastic, etc.) to which an adhesive formulation will adhere well. However, the degree of adhesion is highly dependent upon the details of the surface, the surface preparation, and the details of the curing process. This is discussed in more detail below under failure modes of adhesives.
3.5 Thermal Properties
Temperature can strongly affect the properties of adhesives. In fact, this is one of the major tradeoffs when considering the use of adhesives vs. mechanical fasteners. The following are several important temperature related properties of adhesives.
This is the highest temperature that the cured adhesive can endure for an extended time without deteriorating. For epoxies, this is usually 125 degrees C or higher.2 Adhesive suppliers normally report both the maximum and minimum recommended extended exposure temperatures for their formulations and it is important to consider both extremes in the context of the proposed application.
3.5.2 Glass Transition Temperature (Tg)
Adhesives behave like rubbery materials at high temperature and brittle materials at low temperature due to the change in mobility of the polymer chains in the adhesive with temperature.3 The glass transition temperature (Tg) is the temperature at the mid-point of the transition region between these two types of behavior. The glass transition temperature is an indicator of the maximum usable temperature range because of the drastic change in properties that result when it is crossed. Rubbery materials can become brittle below their Tg.3 Likewise, rigid materials can lose their structural integrity above their Tg.5 While in many cases, the glass transition temperature falls somewhat below the maximum continuous operating temperature, in applications where the bond will be subjected to large stresses, the Tg should be above the maximum projected use temperature.2 Alternatively, if rubbery behavior is desired, the Tg should be below the minimum projected use temperature. Note that the glass transition temperature is a function of the adhesive formulation as well as the cure temperature and schedule. To maximize Tg, the adhesive must be cured at the highest recommended temperature for a sufficient length of time.
3.5.3 Coefficient of Thermal Expansion (CTE)
Materials expand linearly with an increase in temperature. The coefficient of thermal expansion (denoted CTE or a) is the constant of proportionality between the temperature change and the induced thermal strain:
a = e / DT = DL/(L.DT)
The CTEs of adhesives are typically much higher than the structural materials that they are joining (e.g. 30-300 ppm/deg C for adhesives compared to 23 ppm/deg C for aluminum and 7ppm/deg C for BK7). Typically, the designer attempts to match the CTEs of the two substrates as closely as possible and then find an adhesive with a CTE near that value.3 If there is a large mismatch between the CTE of the adhesive and substrate(s), large stresses can result upon temperature variations. If the adhesive has a low Young’s modulus, the stresses resulting from thermal expansion can be lessened. But, in general, low CTE adhesives are desired.
This could perhaps be best described as a long term shape change and is critical for applications where dimensional stability is important. Keep in mind that creep rate is exponentially proportional to temperature and is also influenced by stress and humidity.
3.6 Chemical Properties
This is the resistance of a cured adhesive to degradation by chemical attack. Once again, this is a property that is hard to quantify. Adhesive suppliers will typically include comments such as “excellent chemical and solvent resistance” in their adhesive selection tables and may post some limited data reporting testing against certain solvents. However, a particular formulation may be resistant to a handful of chemicals, but much less resistant to others. Also, the chemical resistance of a particular adhesive is strongly dependent upon the details of its curing and aging. This is discussed in more detail below.
There is no uniformly accepted method for classifying adhesives. Some authors categorize them according to their chemical family, others according to their cure method, others according to their usage. In all cases, there is some overlap among these different classification methods.
Epoxies are available in one- or two-part varieties. Two-part epoxies generally demonstrate good adhesion, high strength, and some degree of resiliency. Their chief limitations are critical mix ratio dependency, long room temperature cure times or high temperature cure requirements, high CTE, and high Young’s modulus. The latter two result in rigid bonds and high stress levels.3 When used in structural applications, they sometimes have filler materials, such as silver powder to increase electrical conduction, aluminum oxide to enhance thermal conduction, or silica powder to act as a thickening agent.6
Available in one- or two-part forms, urethanes (or polyurethanes) form strong, durable bonds to many types of substrates. They are flexible and consequently good for bonding components with differing CTEs. Their environmental resistance is worse than that of epoxies. The upper temperature limit at which they can be used is about 100 degrees C.6
Silicones are elastomers, tend to be somewhat flexible upon cure, and are often used to bond materials with dissimilar thermal expansion coefficients. They are chemically inert and generally function well over a temperature range of about –80 to 204 degrees C. A common type of silicone adhesive is RTV, which stands for “room temperature vulcanizing.” These have become very popular because they are very easy to apply. However, as with all adhesives, cleaning and priming metal surfaces before applying RTV is very important. Silicones are usually used as sealants. They are generally not considered to be structural adhesives, although they are sometimes used to bond lightweight components. Some silicones have significant outgassing both during and post cure. Some have been formulated for low outgassing, such as Dow Corning DC93-500. About 1% shrinkage is typical for silicones. A typical CTE is around 200-300 ppm/degree C. Silicones usually have a tensile strength is of around 30-90 psi (which is why they are seldom used for structural mounting). Cure times depend strongly upon temperature. The following are typical cure schedules: 2 days at 25 deg C, 4 hours at 65 deg C, 1 hour at 100 deg C, 15 minutes at 150 deg C. Some varieties rely on atmospheric humidity to cure and require about 50% relative humidity for thorough curing. Cure is from the surface inward, so thick bonds may require longer times. Silicones are usually available in colors as well as clear and translucent varieties. The thermal conductivity of silicones is usually about 8.4 W/m2-K, but some varieties have additives to increase this value by up to 5 times. This increases their usefulness as a potting compound for electronic components that need temperature stabilization.6
These materials currently dominate the market for optical cements due to their ease of use and fast cure times, which makes them very attractive for high-volume production of optical components. Most UV-cured optical cements are members of the acrylic family and are doped with UV photo-initiators to allow curing upon exposure to a UV light source. The bond strength of UV-cured acrylics is typically considerably lower than those achieved by thermosetting epoxies, but are often sufficient for the intended applications. Suppliers claim that new formulations are beginning to rival the strengths achieved by epoxies.
Cyanoacrylates (e.g. “superglue”) are single-component adhesives that cure by exposure to the alkaline moisture and ionic substances found on most surfaces. For best adhesion to inert or acidic surfaces, a primer may be applied. Cyanoacrylates characteristically have low viscosity and a short cure time to fixate (typically less than 30 seconds). Some have elastomeric additives to increase the adhesive’s flexibility and gap-filling properties. These materials will quickly bond skin, so working with them requires care in application and full eye protection. Cyanoacrylates have several limitations. They have a potential for failure above 71 degrees C, especially in high humidity. Their bond strength on glass or ceramics may decrease with time. In addition, vapor emitted by uncured adhesive may leave a white residue on nearby surfaces.6
In thermosetting adhesives, cementing and alignment is done at room temperature, which is followed by curing at elevated temperature. Often, these adhesives can be cured in few hours at elevated temperature or in several days at room temperature. The manufacturers usually provide recommended cure temperatures and schedules and their recommendations should be followed.
Two-part systems begin cross-linking upon mixing and thus have a short useful life after mixing, but also offer extended shelf lives. Typical room temperature shelf lives are around 6 months to a year at room temperature. The pot life of a 2-part epoxy indicates the length of time between mixing and significant change in viscosity. This can be anywhere from a few minutes to several days. Two-part epoxies cure uniformly throughout the bulk and thus are preferred for thick bonds.9
Single-component systems are easier to work with because they do not require precise mixing of the two components, but their viscosity gradually increases as they are stored. Freezing or refrigerating it can slow this process down.2
In most cases, the initiator for single-component systems is the water vapor in the air and curing relies upon diffusion of the initiator. As such, it can potentially take years for a very thick bond to fully cure.9
In general, both one- and two-part thermosets usually emit some reaction byproducts, which can sometimes be corrosive or flammable, and result in some degree of shrinkage.9
UV-curing adhesives are one-component systems that are cured upon exposure to UV light. The manufacturers instructions should be followed in terms of the wavelength of the UV source (i.e. UV sunlamp, 365 nm source, etc.), the distance from the part to be cured (e.g. 1” or 12” are common), and the duration of exposure. Most UV-curing adhesives recommend a short pre-cure on the order of tens of minutes followed by a long full cure exposure on the order of an hour or two. Uniform irradiation is important to prevent distortions and stress gradients due to uneven curing. 6
Note that generally only small, low mass optics (e.g. up to about 3” diameter) should be mounted with UV adhesives, due to the limitations in bondline thicknesses which can be easily cured without creating too much stress or shrinkage. Acceptable bondline thicknesses are on the order of 0.010” to 0.250”.5
These materials liquefy upon heating to about 120 degrees C. Re-heating can separate bonded elements.
This type of curing process relies upon evaporation of a solvent at an elevated temperature. Consequently, considerable shrinkage occurs. Canada Balsam is an example of such an adhesive.6
Adhesives are also often classified according to their usage. Typical uses include optical bonding (glass to glass), which would call for optically transparent adhesives, optical mounting (metal to glass), which would call for adhesives with low elastic modulus and low CTE in order to accommodate differential expansion due to the differing CTEs of the two substrates, structural bonding, which would call for high strengths (e.g. tensile or shear strengths in excess of 1000 psi),3 locking adjustment threads, or making temporary bonds, etc.
Military systems have extreme reliability requirements and consequently more stringent requirements than most commercial optical devices. In some cases, military systems have very unique requirements based on the field conditions they may encounter. The reader is directed to an excellent paper by D. H. Krevor, et al. Some key points from this paper are highlighted here.
Optomechanical adhesives used in military systems must be able to resist moisture, rain, and extreme temperatures (i.e. -50 to +80 degrees C working temperatures for avionics; more extreme for storage), as well as thermal shock (fast temperature change) and prolonged sunlight/UV radiation exposure. Free radical can be generated by UV exposure and often this requires free-radical scavengers and anti-oxidants to be included in the adhesive formula.
Military adhesives must also be fungus resistant. Fungus-nutrient materials include epoxy resin and polyurethane, so these would be poor choices for military adhesives. Silicone resin is a fungus-inert material.
Military adhesives must also have good chemical and solvent resistance. In particular, they must be resistant to degradation upon exposure to jet fuels, hydraulic fluids, coolants, detergents, soaps, fire-fighting foams, Halon and Halon-replacements, and de-icing solvents. Furthermore, they must resist degradation upon exposure to chemical warfare agents and their decontaminants. They must also resist absorption of these materials, to prevent a prolonged exposure hazard to crew members.
Military adhesives must also withstand extreme environments such as volcanic ash, salt atmospheres, and explosive atmospheres.
Military adhesives must also withstand extreme mechanical stresses – vibration, shock, gunfire, and sudden acceleration. Laminating adhesives are often required to provide viscoelastic damping for vibration abatement.
Edge-seal adhesives used in military liquid crystal displays must be non-porous and impervious to liquid crystal materials (which are typically linear aromatic and aliphatic organics). They must also cure without residual volatiles that could leach into the LCD. Both thermal and UV-cure materials are used.
A low ambient pressure (high altitude) environment requires low-outgassing adhesives. Long burn-in times or bake treatments are generally part of the manufacturing processes for optical systems used in aerospace applications. Differential Scanning Calorimetry (DSC) is a technique that is often used to verify that curing conditions have removed all volatiles.
Because of the extreme temperature range, finding adhesives with low CTEs becomes very important. It is also important to find adhesives that do not have glass transition temperatures within this extreme temperature range because of the radical change in properties such as modulus of elasticity and CTE on either side of this temperature. Temperature cycling above and below the glass transition temperature can be particularly detrimental, causing adhesives to become hazy because of phase separation as a function of molecular weight.10
Space is a rigorous environment in which materials are exposed to extreme vacuum and extreme solar radiation without the benefit of atmospheric filtering. Consequently, resistance to out-gassing as well as resistance to UV exposure is crucial for space-based applications.10 According to NASA, space-qualified materials should have TML < 1.0% and CVCM < 0.1%.4
Medical adhesives are required by law to be solventless (100 percent solids before and after curing) due to concerns about the short-term toxic effects of solvents as well as indications that many of them are carcinogenic. Other requirements for medical adhesives include good adhesion to organic and inorganic surfaces, good wetting properties, compatibility with radiation, chemical, and autoclave sterilization, and fungus- and bacteria-resistance. Many optical adhesive suppliers have a line of adhesives that are qualified for medical use.
While not exhaustive, the following table lists several optomechanical adhesive suppliers and contact information. Many of them specialize in a particular category of adhesives, but several of them have a broad selection of products across different adhesive categories. Most or all of them provide significant amounts of technical information, recommendations, and guidelines regarding their materials on their websites.
There are three primary failure modes for adhesives. First, and most common, is adhesive failure. This occurs when the stresses exceed the strength of the adhesive’s grip upon the substrate. Second, is cohesive failure. This happens when the grip of the polymer molecules to each other in the bulk of the adhesive is exceeded, usually by a shear stress. Finally, and least likely, is fracture of the substrate. This can occur when the bond is so strong that the element that fails is actually the substrate. This is unlikely, since the strengths of adhesives are usually significantly lower than the strengths of typical substrates, but this can occasionally happen. The first two are explained in further detail below. For more detail in failure analysis of adhesives, the reader is directed to a very informative paper by H. F. Woods.
In order for good contact between the adhesive and the substrate surface, the surface tension of the adhesive must be much less than the surface energy of the substrate. An accepted rule of thumb is that there needs to be at least 10 dynes/cm difference. Most optical adhesives have surface tensions of 30 to 35 dynes/cm and most glasses and metals have surface energies in excess of 100 dynes/cm, so wetting to these surfaces is not often problematic. However, optical plastics usually have surface energies between 30 and 40 dynes/cm, so it can be challenging to wet many optical adhesives to optical plastics, so one must select adhesives which are specially formulated for this purpose if plastic bonding is required.1
Even when there is good wetting of the adhesive to the optical surface, the grip of the adhesive can be rather weak due to the high polish of many optical surfaces, so other mechanisms are required for a strong bond.
Optical glasses are usually alkaline and optical adhesives are usually acidic. During cure, the substrate and adhesive will exchange ions, creating a strong ionic bond between them. Very strong bonds can be formed if the pH of the adhesive is between 3 and 4. The acid-base reaction can be left incomplete in UV-cured bonds due to the speed with which the liquid converts to a solid, resulting in weaker adhesion.
The third component of adhesion is the attraction between the adhesive and substrate due to van der Waals forces.
Cohesion is the attraction of the adhesive molecules to each other in the bulk of the adhesive. The two primary processes involved in cohesion are van der Waals forces and crosslinking.
Although they play a role in adhesion, van der Waals forces are predominantly involved in cohesion. These forces impart some chemical resistance to the adhesive.
Thermoset adhesives form chemical bonds between the polymer chains called crosslinks, which are much stronger than van der Waals forces. These also impart chemical resistance to the adhesive bond.
Chemical resistance is generally lower in UV-cured adhesives than in thermoset epoxies. Magyar indicates that this may be a result of the UV initiator causing the long chain molecules to link, but leaving the crosslinks to occur by themselves over a longer period of time. Tests have shown that UV initiated thermoset cements showed much higher chemical resistance if heat-treated at 40 degrees C for 1 hour following their full UV cure than if they did not undergo heat treatment.1
Yoder indicates that the solvent resistance of typical UV-cured adhesives is high, especially after aging for about 3 weeks.6 This may be a result of delayed cross-linking.
If high chemical resistance is desired in an application that demands UV-cure, one might consider the UV-curing epoxy, EPO-TEK UVO 114. Unlike most UV-curing adhesives, which are acrylics, this is an epoxy and exhibits superior chemical and moisture resistance as well as tolerance of high temperature conditions. It also exhibits no significant shrinkage upon cure.
Medical adhesives are specifically formulated to be solvent-free (100 percent solids) and thus are likely to be more chemical resistant than generic adhesives. One example is EPO-TEK 377, which is noted as the most moisture-resistant and chemical-resistant adhesive in Epoxy Technology’s line of medical adhesives.11
Silicones are considered chemically inert and are likely to have very good chemical and solvent resistance.6
There is no simple and clear-cut formula for selecting the best adhesive for any particular application. However, an understanding of the material properties that characterize and distinguish optomechanical adhesives will help the optical engineer make informed choices. Understanding the historical context of optical adhesives and the general classes of optomechanical adhesives, including their strengths and weaknesses will aid the engineer in focusing his or her search for an appropriate adhesive for their application. Some applications have special requirements that limit the available adhesive choices. For such applications, including military optical systems, space based systems, and medical devices, it is crucial not to overlook the details of these sometimes stringent requirements. Familiarization with optomechanical adhesive suppliers and their product offerings, as well as making use of the online tools and telephone technical support that they provide, is helpful in selecting some likely candidates for the desired application. For applications that require chemical and solvent resistance, it is important to understand the failure processes of bonds and the things that can contribute to making an adhesive weak to chemical attack. Finally, it is important to make selection decisions with cost always in mind, so that the most economical and effective solutions are found.
J. T. Magyar, “History of and potential for optical bonding agents in the visible,” Proc. of SPIE Vol. 1535,[1991]
A.L. Delmarsh and R.H. Estes, “Selecting Epoxies for Optical and Fiber Optic Applications,”, Technical Paper GB-56, http://www.epotek.com/ssc-white-papers.asp.
J. G. Daly and D. J. Daly, “Structural adhesives for bonding optics to metals: a study of optomechanical stability,” Proc. SPIE 4444, 177 (2001).
http://outgassing.nasa.gov/
Eric A. Norland, “Techniques in using UV adhesives for optomechanical designs,” Proc. of SPIE Vol. 2542, Optomechanical and Precision Instrument Design, [1995]
P.R. Yoder, Jr., Opto-mechanical Systems Design, 3rd ed., CRC Press, Boca Raton, FL, 2006, pp. 128-140.
Alson E. Hatheway “Analysis of adhesive bonds in optics” Proc. of SPIE Vol. 1998, Optomechanical Design, [1993]
J. Burge, Course notes, OPTI-521 Introductory Optomechanical Engineering, University of Arizona, 2006.
B. Olbert, Presentation: “Adhesive selection & characterization – What you don’t know can kill you,” UASO Engineering Seminar, Aug 2004.
D. H. Krevor, H. N. Vazirani, A. Xu “Effects of military environments on optical adhesives” Proc. of SPIE Vol. 1999, Adhesives Engineering,[1993]
R.H. Estes, “The suitability of epoxy-based adhesives for use in medical devices,” Technical Paper GB-63, http://www.epotek.com/ssc-white-papers.asp.
H. Frederick Woods, “Causes for separation in UV adhesive bonded optical assemblies,” Proc. of SPIE Vol. 1999, Adhesives Engineering, ed.[1993]
Source: https://wp.optics.arizona.edu/optomech/wp-content/uploads/sites/53/2016/10/ClementsTutorial1.doc
Web site to visit: https://wp.optics.arizona.edu
Author of the text: indicated on the source document of the above text
If you are the author of the text above and you not agree to share your knowledge for teaching, research, scholarship (for fair use as indicated in the United States copyrigh low) please send us an e-mail and we will remove your text quickly. Fair use is a limitation and exception to the exclusive right granted by copyright law to the author of a creative work. In United States copyright law, fair use is a doctrine that permits limited use of copyrighted material without acquiring permission from the rights holders. Examples of fair use include commentary, search engines, criticism, news reporting, research, teaching, library archiving and scholarship. It provides for the legal, unlicensed citation or incorporation of copyrighted material in another author's work under a four-factor balancing test. (source: http://en.wikipedia.org/wiki/Fair_use)
The information of medicine and health contained in the site are of a general nature and purpose which is purely informative and for this reason may not replace in any case, the council of a doctor or a qualified entity legally to the profession.
The texts are the property of their respective authors and we thank them for giving us the opportunity to share for free to students, teachers and users of the Web their texts will used only for illustrative educational and scientific purposes only.
All the information in our site are given for nonprofit educational purposes