Brazing with a gas torch differs from gas fusion welding in that the base metal is not melted. In both brazing and braze welding only the filler metal is melted. Joint union (coalescence) is accomplished not by fusion of the base metal but by the unique bonding characteristics of the filler metals used.
In BRAZING, filler metal is distributed between joint surfaces by capillary attraction (the same force that draws water from the roots to the branches of a tree).
Brazing requires close-fitting joint areas to ensure good capillary attraction. Brazing joint clearances are usually less than 0.003 of an inch (thinner than this sheet of paper).
Brazing is similar to gas welding in that it is a two-hand operation (when manually performed with a filler rod). However, the processes differ because with brazing the base metal is never melted. The many heat sources for brazing include oxyacetylene, air-acetylene, oxy-propane, oxy-natural gas, carbon-arc torches, and electric induction and resistance furnaces. Most of the gas outfits are simple and well suited to onsite repair applications.
Because the base metal is not melted, brazing is superior to fusion welding for some applications. It has less effect on the heat treatment (tempering and hardening) of the original metals than fusion welding. Because less heat is required, parts are less likely to warp or burn. The entire joint area is evenly heated to the flow point of the brazing (filler) alloy and the heated base metals melt the filler alloy.
For brazing any given base metal, the temperatures required are higher than for soldering but lower than those for fusion welding. Furthermore, brazing, unlike fusion welding allows you to join almost any combination of metals in a single assembly. For example, assemblies incorporating steel, copper, and brass are extremely difficult to join by fusion welding, but can easily be joined by brazing. Simply select a brazing filler alloy that melts at a temperature lower than that of the metals to be joined. Then choose the metals for the assembly that are best suited for the particular application.
Contrary to popular belief, brazed joints can be as strong as fusion welded joints. The strength is simply achieved in a different way. The filler metal is drawn into the joint by capillary action to form a bond between the closefitting surfaces. Brazed joint strength depends on many factors: the degree to which the brazing alloy fills the joint gap; the strength of the brazing alloy and the base metals themselves; the geometry of the brazed assembly; and the type of joint.
Some simple braze joint designs are illustrated here. Note how the butt-lap, scarf, and lap joint designs provide a larger bonding surface area than in the simple butt joint.
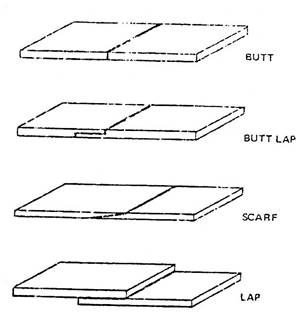
Figure 2 - Braze Joint Designs
This improves the strength of the joint. In general, a sound, brazed joint will be at least as strong as the brazing alloy used, and sometimes even stronger.
A brazing alloy whose ordinary tensile strength is about 60,000 psi can produce a brazed joint with a tensile strength up to 130,000 psi, depending on the joint design and how the filler is applied.
Brazing filler metals are non-ferrous metals that have a melting point above 427°C (800°F), but below the melting point of the base metals. The brass (copper/zinc) or bronze (copper/tin) brazing alloys in rod or wire form are the most widely used. Brazing alloys are also made of copper with other metals such as silver, nickel, cadmium, and phosphorus. And, new alloys of brazing filler metal are being developed so various "exotic" metals such as palladium, titanium, or beryllium can be joined by brazing and braze welding. Brazing is one of the primary methods for joining exotic metals.
Specialised and general purpose fluxes have been developed in powder and paste forms to meet the needs of brazing various alloys and exotic metals. A flux can be a "material used to prevent, dissolve or facilitate removal of oxides and other undesirable substances". In many cases, flux in the form of a core or coating is made part of the filler rod.
Various metals require different types of fluxes. Most fluxes fall into one of several chemical groupings, which include borates, boric acid, alkalies, fluorides and chlorides. Manufacturers have their own trade names for fluxes to be used with different metals. For best results, follow their recommendations for selection and applications.
Powdered flux is usually applied by dipping the heated end of the filler rod into a container of the flux. The powder adheres to the hot rod and melts with the rod during brazing. Some filler rods are supplied already flux-coated, but there are disadvantages to the flux-coated rod. Because the entire rod is coated, sometimes there is too much flux present for a particular braze joint application.
Too much flux can cause a cleanup problem. Also, if the flux-coated rods are not carefully and properly stored, the flux may absorb moisture from the air, and flake or chip. Still another problem occurs if the rod falls or strikes a hard surface, chipping the flux coating.
Powdered flux also can be applied by heating the base metal slightly and sprinkling the powder liberally over the joint area. The powdered flux will partly melt and adhere to the base metal. Flux in liquid or paste form can be brushed over the joint area with a small brush. And, in some new production techniques, fluxes are replaced by the use of special gas atmospheres which surround the parts being brazed and effectively overcome oxidation.
The joint to be brazed must be designed so the two joint edges fit closely. In maintenance brazing this can be difficult at times, but the better the fit-up, the stronger the joint. And, whenever possible, the base metal should be mechanically cleaned to remove all dirt, paint, oil and grease.
Do not rely on the flux for that purpose, remember the primary function of flux is to remove oxides and prevent them from re-forming.
Dark lens goggles or glasses should be worn to protect your eyes during brazing. The first step in brazing is to adjust the torch to the proper flame. When brazing carbon steels, use a neutral oxyacetylene flame. For cast iron, adjust the flame to a slightly oxidising condition by increasing oxygen or decreasing acetylene flow. This type of flame helps remove the graphite from the surfaces of cast iron. Use a small, gentle flame for thin materials and a larger flame for the thicker materials.
For brazing, the flux is usually applied directly to the joint. In braze welding, the brazing rod is warmed and dipped in the flux. The flame is applied to the joint area, heating both edges or surfaces to a dull red colour. Do not overheat the base metal. If the base metal becomes too hot, the zinc in a brass filler rod will burn off and this may produce toxic fumes. Too much heat is indicated when the joint area turns copper in colour. Some brazing flux compounds are specially formulated so they melt when the proper brazing temperature is reached.
While heating the metal, keep the end of the brazing rod in or near the torch flame to preheat the rod. This helps the rod melt more easily when touched to the hot base metal, and makes it easier to control the amount of filler metal deposited. When the base metal has been heated to dull red, bring the brazing rod into contact with the dull red area. Maintain uniform heating in the base metal by using a smooth, uniform torch motion. The brazing rod will quickly melt and flow over or between the joint surfaces.
The width of a braze bead is determined by the width of the portion of the base metal that is heated enough to melt the filler metal. The filler metal will only flow on and adhere to a base metal surface that is free of oxides and is at the correct temperature. Moving the torch flame in a particular direction causes the filler metal to follow in that same direction. As a guideline, the width of the braze bead should be just a little wider than a normal fusion weld on the same thickness of metal.
The finished brazed joint should have the appearance of adequate adhesion to the base metal. The filler metal should seep all through the brazed joint and appear underneath.
A white deposit (like white soot) along the edge of the brazed or braze welded joint indicates an overheated joint. Discoloration of the braze filler metal in the joint also indicates overheating. The best brazed joint will show a colour similar to the brazing filler metal used. A glassy substance around the joint indicates excess flux was used. This should be removed as soon as possible.
The same safety procedures, we observe when gas welding, apply to brazing. Because of the broad mix of metals suitable to brazing there are more fumes to be aware of. Brazing galvanised steel emits hazardous fumes. It is advisable to wear a protective mask when brazing at all times.
When a hot body is brought into contact with a cold body heat is transferred from the hot body to the cold body. There is a considerable difference between temperature and quantity of heat. A white-hot spark from a grinding operation may be at a temperature as high as 1500°C, yet no discomfort is felt when it contacts with a cold hand. A very painful scald will result when water at 100°C contacts the hand.
The unit of heat energy is the joule, written J for short. The joule (J) is a very small unit of energy and in practice the kilojoule (kJ) and the megajoule (MJ) are used.
1,000 J = 1 kilojoule (kJ)
1,000,000 J = 1 megajoule (MJ)
Hard soldering is a general term used to cover ‘brazing’ and ‘silver soldering’ which are very similar thermal joining processes.
In this process, as in soft soldering, melting or fusion of the parent metals to be joined does not take place, which means that a suitable filler material which has a lower melting point than that of the parent metal is employed.
There are a number of alloys other than the familiar TIN/LEAD alloys which can be used as SOLDER. They do not possess the low melting point of the soft solders, but they have other properties such as HIGHER STRENGTH, which makes them preferable for certain jobs.
For many years bicycle frames have been made by joining alloy steel tubes to the brackets by a BRAZING operation. In this case the solder is a grade of BRASS (hence the name BRAZING) which usually consists of 60% ZINC and 40% COPPER - this alloy melts at about 850°C, which is much higher than the soft-soldering temperatures. Such high melting point solders are called HARD SOLDERS, and hard soldered or brazed joints are much stronger than ordinary soft soldered ones.
Brazing is defined as ‘a process of joining metals in which molten filler metal is drawn by capillary attraction into the space between closely adjacent surfaces of the parts to be joined. In general the melting point of the filler metal is above 500°C.' In this respect, and broadly in its application, brazing lies between soft soldering and fusion welding.
The success of all brazing operations depends on the following general conditions:
The above principles also apply to the process of silver soldering. When a surface is 'wetted' by a liquid, a continuous film of the liquid remains on the surface after draining. This condition is essential for brazing and silver soldering, for the flux having removed the oxide film completely 'wets' the surfaces of the joint faces. This wetting action by the flux assists spreading and feeding of the filler material to the CAPILLARY SPACES, leading to the production of completely filled joints.
AN IMPORTANT FEATURE OF HARD SOLDERING IS THAT THE FILLER MATERIAL IS DRAWN INTO THE JOINT AREA BY CAPILLARY ATTRACTION. For anyone combination of solid and liquid, the smaller the gap the deeper is the capillary penetration. The principles of capillary flow, illustrated in Figure 3 are explained below.
Consider a clean piece of glass plate with a small droplet of water lying on it as shown in Figure 3(a).
If another piece of clean glass plate is laid on the first piece and made to slide until its edge is brought into contact with the water droplet, some of the water will immediately flow into the very narrow gap between the mating surfaces, for an appreciable distance; by capillary attraction.
Exactly the same effect occurs when a joint is hard soldered, except that instead of glass plates there are the mating surfaces of the components to be joined and in place of water, molten filler material.
The effects of 'capillary attraction' are shown schematically in Figure 3(b). Four pairs of glass plates are arranged with increasing gaps A, B, C, and D standing in a shallow bath of coloured liquid. The liquid has risen the highest between the pair with the smallest gap, A. In the right-hand pair liquid has risen to the top of the back edge but only slightly at the front, wide gap D.
The effectiveness of capillary attraction is governed by the maintenance of appropriate joint clearances:
The mating surfaces should be parallel. There should be no break in the uniformity of clearance.
If a break occurs due to a widening or closing, then capillary flow will stop in that vicinity and may not go beyond it.
As shown in Figure 3(c) CAPILLARY ATTRACTION CAN BE EXPECTED TO DRAW THE FILLER METAL AROUND A CORNER WHEN THE CONDITIONS ARE CORRECT.
Capillary flow may easily be stopped and cut off from the rest of the joint by a chamfered corner, as shown at Figure 3(d).
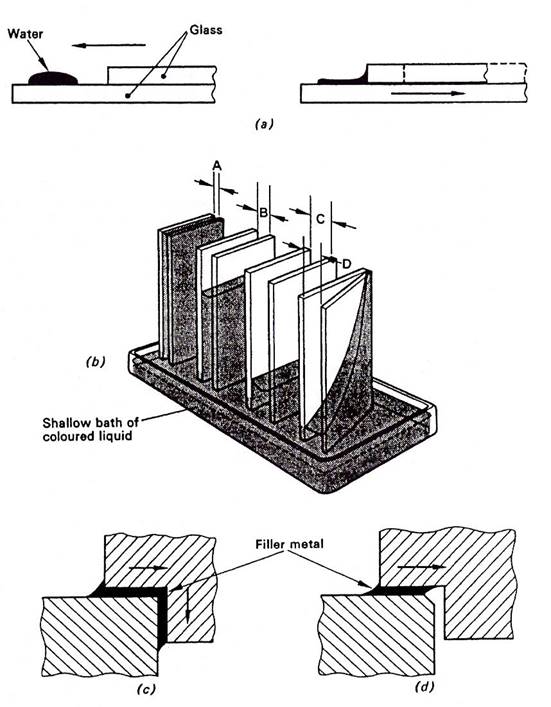
Figure 3 - Capillary Flow
The following common metals and their alloys may be joined by the process of brazing:
Copper and copper based alloys;
Mild steel, carbon steel and alloy steels;
Stainless steels and stainless irons;
Malleable and wrought iron;
Nickel-base alloys;
Aluminium and certain aluminium alloys.
Metals and alloys of a dissimilar nature can also be brazed together, for example: copper to brass; copper to steel; brass to steel; cast iron to mild steel; mild steel to stainless steel.
For most metals the brazing alloys used are normally based on COPPER-ZINC ALLOYS and are dissimilar in composition to the parent metals to which they are applied. COPPER is commonly used as a brazing material for the flux-free brazing of MILD STEEL in reducing atmosphere furnaces.
Brazing alloys may be classified into three main types:
These are more expensive than the normal brazing alloys because they contain a high percentage of SILVER, but they offer the advantages of producing very strong and ductile joints of much lower temperatures.
British Standard 1845 |
Composition Percentage |
Approximate Melting Range |
|||
Silver |
Copper |
Zinc |
Cadmium |
||
3 |
49 to 51 |
14 to 16 |
15 to 17 |
18 to 20 |
620-640 |
4 |
60 to 62 |
27.5 to 28.5 |
9 to 11 |
- |
690-735 |
5 |
42 to 44 |
36 to 38 |
18.5 to 20.5 |
- |
700-775 |
Type 4 possesses a high conductivity and is, therefore, very suitable for making electrical joints. It is the most expensive because of its high silver content. Type 3 is extremely fluid at brazing temperatures which makes it ideal when brazing dissimilar metals. A low melting point alloy. |
|||||
Table 1 - Composition of silver Solders
Silver solders are very free-flowing at brazing temperature. Their tensile strength is in the region of 500 MN/m², and because of the low brazing temperatures required, have very little heat-effect upon the properties of the parent metals.
By using silver solders it is possible to increase the speed of brazing and to eliminate or to limit 'finishing' operations. One of its main applications is for delicate work in which small neat joints are essential.
Borax-type fluxes are not suitable for silver soldering.
Because of their low melting temperatures silver solders should not be used with high-melting brazing fluxes based on BORAX or BORIC ACID. These fluxes are not sufficiently fluid for silver soldering temperatures below 760°C. POTASSIUM FLUOBORATE can be used as a suitable flux; it is very active and completely molten at 580°C.
An efficient flux should melt at a temperature at least 50°C lower than the melting point of the brazing material and retain its activity at a temperature at least 50°C above the melting temperature of brazing alloy being used. Because of this factor it is advisable to use the proprietary fluxes which are available on the market. Table 1 gives the compositions of three silver solders together with their melting ranges.
Filler materials which contain PHOSPHORUS are usually referred to as 'self-fluxing brazing alloys'. These alloys contain SILVER, PHOSPHORUS and COPPER or, COPPER and PHOSPHORUS, the former possessing a lower melting range. The outstanding feature of these alloys is their ability to braze copper in air without the use of a flux. When melted in air the products of OXIDATION form a fluid compound which acts as an efficient flux. A suitable flux is required when brazing copper-based alloys.
British |
Composition Percentage |
Approximate Melting |
||
Silver |
Phosphorus |
Copper |
||
6 |
13 to 15 |
4 to 6 |
Balance |
625-780 |
7 |
- |
7 to 7.5 |
Balance |
705-800 |
Table 2 - Composition of Brazing Alloys Containing Phosphorus
Brazing alloys containing phosphorus are only effective if melted in an oxidising atmosphere and should only be used when brazing COPPER and all COPPER-BASED alloys excepting those containing more than 10% NICKEL.
Nickel, nickel-base alloys and ferrous metals should not be brazed with these phosphorus-containing alloys. Although they will 'wet' and flow on such materials, they form brittle compounds which weaken the joint.
By comparison, the TENSILE strength of the silver/phosphorus/copper brazing alloys is about 450 MN/m² whilst the higher melting point copper/phosphorus alloys is much less at 350 MN/m².
These brazing alloys find their greatest application in resistance brazing operations, in refrigerator manufacture, electrical assemblies (electric motor armatures), for brazing seams and fittings in domestic copper hot-water cylinders, and in plumbing. Table 2 gives the composition and melting ranges of two common brazing alloys containing phosphorus.
The oldest and best known method of brazing involves the use of brazing brasses or 'brazing spelters', using BORAX as a flux.
These alloys melt at much higher temperatures than the silver solders and the phosphorus-containing brazing alloys, but produce sound joints having TENSILE strengths between 400 MN/m² and 480 MN/m². The composition and melting ranges of three common brazing spelters are shown in Table 3 in which it can be seen that increasing the zinc content decreases the melting range. This makes it possible to make a joint in 60/40 brass using a 50/50 brass as the brazing alloy. Conversely, it is important that a brass to be joined by brazing should have a high copper content compared with the brazing alloy used.
This group of copper alloys tends to lose zinc by vaporisation and oxidation when the parent metal is heated above 400°C. This loss of zinc produces relatively higher tensile strength. The brazing alloys containing a high percentage of zinc, therefore, produce joints of the lowest strength.
British |
Composition Percentage |
Approximate Melting |
|
Copper |
Zinc |
||
8 |
49 to 51 |
Balance |
860-870 |
9 |
53 to 55 |
Balance |
870-880 |
10 |
59 to 61 |
Balance |
885-890 |
Type 8 is used for medium strength joints, whilst the strongest joints can be produced by using type 10. |
|||
Table 3 - Composition of Brazing Spelters
There is a distinction between the brazing of ALUMINIUM and the brazing of other metals. For aluminium, the filler material is one of the ALUMINIUM ALLOYS having a melting point below that of the parent metal.
The various grades of commercially pure aluminium alloys containing 1¼% MANGANESE, and certain ALUMINIUM/MAGNESIUM/ SILICON alloys can be successfully joined by brazing.
Aluminium/magnesium alloys containing more than 2% magnesium are difficult to braze, as the oxide film is tenacious and difficult to dissolve with ordinary brazing fluxes.
Borax-base fluxes are unsuitable for brazing aluminium and its alloys, but many proprietary fluxes are available, and these are basically mixtures of the ALKALI METAL CHLORIDES and FLUORIDES. A standard aluminium brazing flux contains essentially chlorides of SODIUM, POTASSIUM and LITHIUM.
These fluxes readily absorb moisture when exposed to the atmosphere, i.e. are 'HYGROSCOPIC'. It is important, therefore, that all lids or covers be firmly replaced on flux containers, when not in use, to prevent rapid deterioration of their contents. Care should be taken when handling aluminium brazing fluxes containing fluorides, as personal contact may result in skin complaints, and toxic effects may follow the inhalation of fumes from these compounds.
Source: http://local.ecollege.ie/Content/APPRENTICE/liu/sheetmetal_notes/module3/Brazing%20Hard%20Soldering_M3_U6.doc
Web site to visit: http://local.ecollege.ie
Author of the text: indicated on the source document of the above text
If you are the author of the text above and you not agree to share your knowledge for teaching, research, scholarship (for fair use as indicated in the United States copyrigh low) please send us an e-mail and we will remove your text quickly. Fair use is a limitation and exception to the exclusive right granted by copyright law to the author of a creative work. In United States copyright law, fair use is a doctrine that permits limited use of copyrighted material without acquiring permission from the rights holders. Examples of fair use include commentary, search engines, criticism, news reporting, research, teaching, library archiving and scholarship. It provides for the legal, unlicensed citation or incorporation of copyrighted material in another author's work under a four-factor balancing test. (source: http://en.wikipedia.org/wiki/Fair_use)
The information of medicine and health contained in the site are of a general nature and purpose which is purely informative and for this reason may not replace in any case, the council of a doctor or a qualified entity legally to the profession.
The texts are the property of their respective authors and we thank them for giving us the opportunity to share for free to students, teachers and users of the Web their texts will used only for illustrative educational and scientific purposes only.
All the information in our site are given for nonprofit educational purposes