Description of Modern Chrome Plating Processes
By: George D. Monninger
Introduction
Chrome plating is a chemical process in which a solid part such as steel, aluminum, or plastic is coated with a layer of chromium. Chrome plating can be performed for a variety of reasons, most commonly protection and decoration. Chrome is commonly desirable in the automotive and commercial industries due to its opulence and corrosion resistance properties.
In most cases, chrome plating is a complex process involving several steps in order to achieve the desired finish. The most common practice used today for chrome plating involves using a series of chemical vats that are used to submerge the part to be chrome plated. Chrome plating involves copper, nickel, and chromium. The methods and procedures of chrome plating drive up the cost of a material, while adding a number of protective and opulent traits.
Applicable Materials
The chrome plating process can be used in conjunction with a variety of materials. Although chrome is most commonly associated with metals, plastics can also be chrome plated. This method is known as plating on plastics. Plating on plastics relies on the same method used to chrome metal pieces. In fact, there is virtually no difference in the process whatsoever. With this in mind, just about any solid material can be chrome plated, given the necessary equipment.
There are some alternative methods to making a material appear chrome. One particular example of this is the “do it yourself” chrome kit. A kit such as this typically contains three or four aerosol cans, each with different ingredients. Typically, the cans consist of a primer, chromium with nickel, and clear coat. This is truly not chrome plating, because the chromium and nickel does not bond to the surface material. It is simply adhered to it. (“The Web is Where You Study in!”)
Chroming Process
In order to achieve the goal of chroming a metal or plastic part, a series of relatively involved steps are required. It is imperative that any metal or plastic subjected to chrome plating is stripped and cleansed of any impurities, as this may result in a poor finish or bad quality. In fact, the slightest bit of dirt or debris will result in rejection of the chrome plating process.
Once a material has been stripped of impurities and is shaped to its desired feature, it is sent through a series of processes where various chemicals are applied to it. The first coat of material applied to the surface is a layer of copper. The copper acts as the primary corrosion protectant in the process. After the copper is applied, the part or material is buffed to a brilliant shine to remove excess copper that has not totally bonded properly. Again, cleaning here is a vital part of the process.
After cleaning, the part or material is subjected to a layer of nickel. The nickel provides the silver-like color of the chromed material.
The final step of the process is adding a layer of chromium. The chromium is actually just a glossy protective layer that covers the already shiny layer of nickel. After which the material is polished and cleaned once again. (Vernes Chrome Plating)
Chemical Deposition
The diagrams below (figures 1 and 2) illustrate the electroplating process, specifically the final stage involving the application of chromium. In order to properly chrome materials, a large supply of water, chemical substrates, and an industrial power supply is needed. Often, entire warehouses are dedicated solely to chrome plating. In the diagram, the component is submerged into the chromium solution. The diagram illustrates the conductivity of the chromium based on the electric conductivity and the chemical reaction process.
The vats contain an aqueous solution of the chemicals. When an electric charge is applied to the bath, the chemical becomes oxidized and is then attracted to the surface to be coated. One side of the bath is positively charged (anode), while the other is negative (cathode). This helps draw the current through the vat, ensuring the correct amount of chemical deposition onto the surface. The vats are made from non-conductive material in order to avoid electric interference.
When non-conductive materials such as plastic are chrome plated, the plastic itself is charged with electricity in order to draw the chemical particles to its surface. Besides this minor difference, the chemical reactions process are identical both with metal and plastic. (“Greening the Chrome Plating Industry”)
Figure 1:
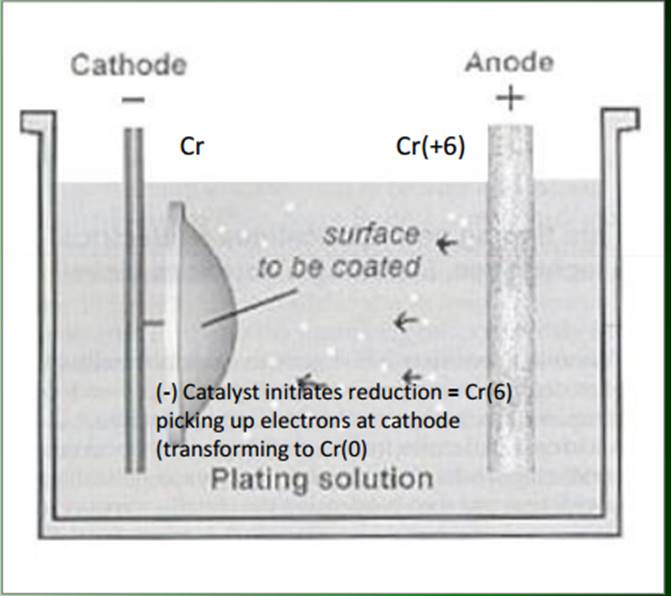
Image From: http://www.wmich.edu/mfe/mrc/greenmanufacturing/pdf/chromeplatinginfo.pdf
Figure 2:
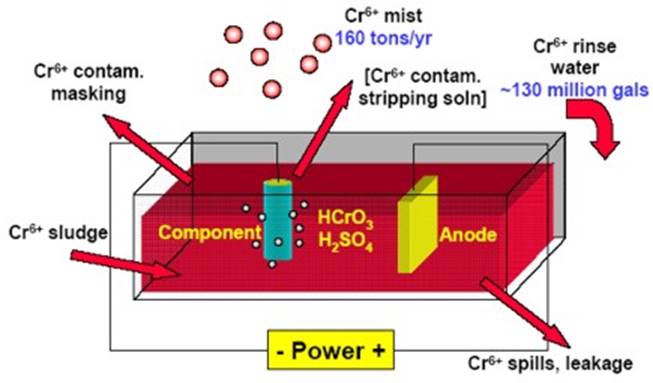
Image From: http://www.ustudy.in/node/3221
Environmental Concerns
Due to a number of negative effects on the environment, a number of restrictions have been placed on chrome plating processes. OSHA and the FDA both mandate strict regulations pertaining to rinse water disposal and chemical waste. Manufacturers who perform chrome plating must completely filter the chromium, nickel, and copper content before deposing of it through the septic system. This is the final step in the process, and perhaps the most important in terms of the environment.
Because of these strict regulations, it is much cheaper for chrome plating industries to operate overseas, where such pollution laws are not enforced quite as heavily. In overseas operations, a much higher concentration of chromium is applied to the material. The waste water is often not filtered properly and returned to the environment tainted. This can cause a serious problem for wildlife and the water table system in the area, leading to severe illnesses such as cancer. (“Greening the Chrome Plating Industry”)
Conclusion
Chrome plating is a process that protects and improves the appearance of a metal or plastic material. Chrome is highly desirable due to its deep luster and reflective properties. In order for a material to be chromed properly, a series of industrial type vats are required. Although the chemical reaction process is the most involved step of the process, constant cleaning of the materials is vital. Once a product is finished, proper disposal of waste water is imperative in order to prevent pollution in the environment.
Works Cited
"Chrome Plating Process | Nickel and Copper Plating, Polishing and Buffing." Chrome Plating
Process | Nickel and Copper Plating, Polishing and Buffing. N.p., n.d. Web. 21 Mar. 2014.
Johnson, Matthew. "Greening The Chrome Plating Industry." N.p., n.d. Web. 21 Mar. 2014.
"The Web's Where You Study In!" Chrome Plating. N.p., n.d. Web. 21 Mar. 2014.
Source: https://sites.psu.edu/georgedmonningereportfolio/wp-content/uploads/sites/12018/2014/04/Eng_202C_chrome_plating.docx
Web site to visit: https://sites.psu.edu
Author of the text: indicated on the source document of the above text
If you are the author of the text above and you not agree to share your knowledge for teaching, research, scholarship (for fair use as indicated in the United States copyrigh low) please send us an e-mail and we will remove your text quickly. Fair use is a limitation and exception to the exclusive right granted by copyright law to the author of a creative work. In United States copyright law, fair use is a doctrine that permits limited use of copyrighted material without acquiring permission from the rights holders. Examples of fair use include commentary, search engines, criticism, news reporting, research, teaching, library archiving and scholarship. It provides for the legal, unlicensed citation or incorporation of copyrighted material in another author's work under a four-factor balancing test. (source: http://en.wikipedia.org/wiki/Fair_use)
The information of medicine and health contained in the site are of a general nature and purpose which is purely informative and for this reason may not replace in any case, the council of a doctor or a qualified entity legally to the profession.
The texts are the property of their respective authors and we thank them for giving us the opportunity to share for free to students, teachers and users of the Web their texts will used only for illustrative educational and scientific purposes only.
All the information in our site are given for nonprofit educational purposes