1. Vulcanization and Curing
Vulcanization or curing is the process in which the chains are chemically linked together to form a network, thereby transforming the material from a viscous liquid to a tough elastic solid. Strength and modulus increase, while set and hysteresis decrease. Various curing systems are used to vulcanize different types of elastomers,
1.1 Sulfur Curing
The most widely used vulcanizing agent is sulfur. For sulfur to effectively crosslink a rubber, an elastomer must contain double bonds. General purpose diene elastomers such as BR, SBR, NR, and IR meet this basic requirement.
Two forms of sulfur are used in vulcanization: soluble (crystals of S8 rings) and insoluble (amorphous, polymeric sulfur). Sometimes, in compounds containing high levels of sulfur, insoluble sulfur is used to prevent sulfur blooming, a process by which the sulfur migrates to the surface of a compound and crystallizes there. Blooming can occur when large amounts of soluble sulfur are used, because at high mixing temperatures, the solubility of S8 is high, enabling large amounts to dissolve, but upon cooling the solubility decreases. When the solubility limit is reached, excess sulfur blooms to the surface. Sulfur bloom reduces the "tack" of a rubber compound, a necessary property if layers of rubber are to be plied up to make a composite structure, such as a tire. Insoluble sulfur does not bloom because it disperses in rubber as discrete particles, which cannot readily diffuse through the rubber. However, above 120°C, insoluble sulfur transforms into soluble sulfur. Thus, mixing temperatures must be kept below 120°C to take advantage of the bloom resistance of insoluble sulfur.
Crosslinking with sulfur alone is quite inefficient and requires curing times of several hours. For every crosslink, 40 to 55 sulfur atoms are combined with the rubber. The structure contains polysulfide linkages. Much of the sulfur is not involved in crosslinks between chains. Moreover, such networks are unstable and have poor aging resistance.
To increase the rate and efficiency of sulfur crosslinking, accelerators are normally added. These are organic bases
Often, a combination of accelerators is used to obtain the desired scorch resistance and cure rate. Generally, if two accelerators of the same type are combined, then cure characteristics are approximately the average of those for each accelerator alone. However, there is no general rule when combining accelerators of different types. Moreover, the type of accelerator is much more important than the level of accelerator in controlling scorch time. Although increased levels of accelerator increase the degree of crosslinking attained, generally accelerator concentration has only a small effect on scorch time.
Accelerated sulfur curing is more efficient when the activators zinc oxide and stearic acid are added. It is thought that these additives combine to create soluble zinc ions that activate intermediate reactions involved in crosslink formation.
1.2 Influence of Crosslink Density
Mechanical properties of an elastomer depend strongly on crosslink density. Modulus and hardness increase monotonically with increasing crosslink density, and the material becomes more elastic, less hysteretic. Fracture properties, such as tear and tensile strength, pass through a maximum as crosslinking is increased. To understand this behavior, it is helpful first to consider fracture in an uncrosslinked elastomer, and then to discuss changes in the mechanism of fracture as crosslinks are introduced.
When an uncrosslinked elastomer is stressed, chains may readily slide past one another and disentangle. At slow rates, fracture occurs at low stresses by viscous flow without breaking chemical bonds. The effect of a few crosslinks is to increase the molecular weight and creating branched molecules. It is more difficult for these branched molecules to disentangle and hence, strength increases. As crosslinking is increased further, the gel point is eventually reached when a three dimensional network forms. A gel cannot be fractured without breaking chemical bonds. Thus, strength is higher at the gel point, because chemical bonds must be ruptured to create fracture surface. However, strength does not increase indefinitely with more crosslinking.
When an elastomer is deformed by an external force, part of the input energy is stored elastically in the chains and is available (released upon crack growth) as a driving force for fracture. The remainder of the energy is dissipated through molecular motions into heat, and in this manner, is made unavailable to break chains. At high crosslink levels, chain motions become restricted, and the "tight" network is incapable of dissipating much energy. This results in relatively easy, brittle fracture at low elongation. Crosslink levels must be high enough to prevent failure by viscous flow, but low enough to avoid brittle failure.
Both the level and type of crosslinking are important. When curing with sulfur, the type of crosslinks depends on (1) sulfur level (2) accelerator type (3) accelerator/sulfur ratio and (4) cure time. Generally, high accelerator/sulfur ratio and longer cure time increase the number of monosulfidic linkages at the expense of polysulfidic ones. Vulcanizates containing predominately monosulfidic crosslinks have better heat stability, set resistance, and reversion resistance than those with polysulfidic links. This is attributed to greater stability of C-S bonds compared to S-S bonds. On the other hand, compounds containing a high proportion of polysulfidic crosslinks possess greater tensile strength and fatigue cracking resistance compared to compositions with monosulfidic links. This is thought to be due to the ability of S-S bonds in polysulfidic linkages to break reversibly, thereby relieving locally high stresses that could initiate failure.
1.3 Other Cure Systems
Peroxides are another type of curing agent for elastomers. Unlike sulfur vulcanization, carbon-carbon double bonds are not required for peroxide curing and thus, peroxides may be used to crosslink saturated elastomers, e.g., ethylene-propylene copolymers,chlorinated polyethylene, chlorosulfonated polyethylene, and silicone rubber. In addition, peroxides readily crosslink diene elastomers. Peroxide curing takes place via a free-radical mechanism and leads to carbon-carbon crosslinks, which are quite stable. The crosslinked materials show good aging resistance and low compression set.
Some elastomers, particularly polychloroprene, can be crosslinked with the metal oxides ZnO and MgO. Generally, mixtures of ZnO and MgO are used because ZnO by itself is too scorchy and MgO alone is inefficient.
2. Reinforcement
Particulate fillers can increase the strength of an amorphous rubber more than 10-fold. For a filler to cause significant reinforcement, it must possess high specific surface area, i.e., the particles must be small, less than 1 µm in size. Small particles have large surface area to interact with the rubber and close particle-to-particle spacing in the compound. Two types of fillers that are most effective for reinforcing rubber are carbon black and silica. They can be produced with a primary particle size as small as 100 °A, corresponding to a surface area of a few hundred m2 per gram of filler.
Before addition to rubber, carbon black aggregates are "clumped" together as so-called agglomerates. To provide the greatest reinforcement, these black agglomerates must be broken down into aggregates and thoroughly dispersed in the rubber. This requires mixing at high shear stress. The viscosity of the rubber must not be too low, or the shear stresses will be insufficient to break apart the filler agglomerates. Thus, when oil is to be added to a stock, it should be added toward the end of the mixing cycle, after the carbon black has been well dispersed.
Besides enhancing strength, carbon black also improves processability by greatly reducing melt elasticity. This allows shaping operations, such as extrusion and calendering, to occur with less shrinkage and melt distortion.
Carbon black is most effective for strengthening non-crystallizing elastomers such as SBR. Strain-crystallizing elastomers such as NR already have a self-reinforcing mechanism. Indeed, unfilled and black-filled NR vulcanizates have comparable tensile strengths, although the latter have improved resistance to tearing and abrasion.
3. Anti-Degradants
Oxygen and ozone can react with elastomers and alter network structure by causing chain scission and/or crosslinking. Antioxidants and antiozonants, which can function chemically or physically, have been developed to inhibit the action of these reactive components of air.
Chemical protectants are capable of reacting with the degradant or interfering with the chain of reactions that otherwise would culminate in degradation of the rubber. The most common types are aromatic amines, phenolics, and phosphites. The first type is staining, while the other two are not. Physical protectants function by migrating, i.e., blooming, to the rubber surface and providing a barrier to attack by degradants. Macrocrystalline waxes, which are mixtures of alkanes, isoalkanes, and cyclo-aliphatic hydrocarbons, are commonly used. The rate and extent of blooming are important and depend on the level of compatibility with the elastomer.
3.1 Ozone Attack
Ozone, even when present in the atmosphere at only a few parts per hundred million, readily cleaves carbon-carbon double bonds in elastomers. As a result, an unsaturated rubber, exposed to ozone in the strained state, quickly develops surface cracks. The severity of cracking increases rapidly if the applied strain is above a small threshold level, of the order of 10%. Para-phenylenediamines (PPDs) are effective in reducing ozone cracking in diene rubbers and there is good evidence that they react directly with ozone, competing with the ozone-rubber reaction. However, there are no additives that enable unsaturated elastomers to resist ozone as well as saturated ones.
3.2 Oxidation
In general, the reaction of oxygen with elastomers causes both chain scission and crosslinking. If chain scission dominates during aging, the elastomer softens and eventually may become sticky. This is the usual behavior of unfilled NR and IIR vulcanizates. However, most technical elastomer compounds eventually harden and embrittle during oxidation, a consequence of the dominant crosslinking reactions. For some compounds, in the early stages of oxidation, there is a fortuitous equality in the extent of chain scission and crosslinking, such that modulus does not change. Nonetheless, the altered network now contains increased chain-end defects, and strength and elongation are reduced.
A concentration of only 1 to 2% of reacted oxygen is normally sufficient to cause severe deterioration in an elastomer. The principal mechanism of oxygen attack involves free radical reaction. The first step is the creation of macroradicals as a result of hydrogen abstraction from rubber chains by a proton acceptor. Oxidation continues by reaction of macroradicals with oxygen and the subsequent formation ofperoxy radicals and hydroperoxides, which are readily detected by infrared spectroscopy. Oxidation is accelerated by heat, exposure to ultraviolet light, and the presence of some metals, notably copper, cobalt, and manganese. Also, stress hastens oxidation. For sulfurcured vulcanizates, the oxidation rate increases as sulfur content increases. It is believed that the allylic crosslink site is particularly susceptible to oxidation.
Antioxidants are employed to slow oxidation. They fall into two classes, with different functions. The first type, called preventive antioxidants, react with hydroperoxides to form harmless, non-radical products. The second type, chain-breaking antioxidants, destroy peroxy-radicals that would otherwise propagate.
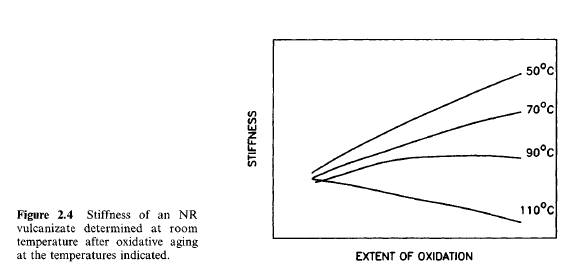
Usually, accelerated aging tests are used to determine the resistance of a vulcanizate to oxidation. However, caution should be used in attempting to infer long-term aging performance from short-term tests carried out at temperatures much higher than the service temperature. The reason is well illustrated in Fig. 1. A black-filled NR vulcanizate was oxidized at various temperatures, while the degree of oxidation was determined directly by the quantity of oxygen absorbed. Stress-strain measurements were carried out on the aged samples. Stiffness at room temperature is plotted schematically against oxygen uptake at various temperatures. It is noteworthy that, when aged at 50°C, the vulcanizate stiffened, while when it was aged at 110°C, it softened. Clearly, in this case, relative rates of chain scission and crosslinking depend onaging temperature.
1
Source: http://www.uobabylon.edu.iq/eprints/eprint_10_30620_726.doc
Web site to visit: http://www.uobabylon.edu.iq
Author of the text: indicated on the source document of the above text
If you are the author of the text above and you not agree to share your knowledge for teaching, research, scholarship (for fair use as indicated in the United States copyrigh low) please send us an e-mail and we will remove your text quickly. Fair use is a limitation and exception to the exclusive right granted by copyright law to the author of a creative work. In United States copyright law, fair use is a doctrine that permits limited use of copyrighted material without acquiring permission from the rights holders. Examples of fair use include commentary, search engines, criticism, news reporting, research, teaching, library archiving and scholarship. It provides for the legal, unlicensed citation or incorporation of copyrighted material in another author's work under a four-factor balancing test. (source: http://en.wikipedia.org/wiki/Fair_use)
The information of medicine and health contained in the site are of a general nature and purpose which is purely informative and for this reason may not replace in any case, the council of a doctor or a qualified entity legally to the profession.
The texts are the property of their respective authors and we thank them for giving us the opportunity to share for free to students, teachers and users of the Web their texts will used only for illustrative educational and scientific purposes only.
All the information in our site are given for nonprofit educational purposes