HIGH POLYMERS
Definition of polymers: A polymer is a large molecule of high molecular weight obtained by the chemical interaction of many small molecules of low molecular weight of one or more type. The process of manufacture of a polymer is called the polymerization.
Monomers: Small molecules of low molecular weight, which combine to give a polymer, are called monomers.
Degree of polymerization: The number of monomers used in the process is called degree of polymerization.
Functionality: The total number of functional groups or bonding sites present in a monomer molecule is called the functionality of the monomer.
Classification of polymers:
Egs. Wood, cellulose, Jute, Cotton, Wool, Silk, Proteins, Natural rubber etc.
Egs: Nylon66, PVC, Polystyrene, Teflon, Plexiglass, Polyesters, Polyethylene etc.
1. Thermoplastic polymers: egs: PVC, Polyethylene etc.
2. Thermosetting polymers: egs: Bakelite, Urea-Formaldehyde etc.
III. Based on their mechanism of polymerization they are classified into
1. Addition polymers: egs: PVC, Polyethylene etc.
2. Condensation polymers: egs: Nylon66, Polyester etc.
IV. Based on their properties they are classified into
1. Elastomers egs; Natural rubber
2. Fibres egs: Jute, Wood, Silk etc
3. Resins egs: Urea- Formaldehyde, Epoxy resins, Phenol- Formaldehyde etc.
4. Plastics egs: Plexiglass, PVC, Teflon etc.
Polymerization: Is the process of conversion of low molecular weight substances into high molecular weight substances with or without the elimination of by products such as HCl, H2O, NH3 etc.
Types of polymerization:
Egs: 1. Formation of polythene.
![]() n CH2= CH2 [- CH2 – CH2 -]n
n CH2= CH2 [- CH2 – CH2 -]n
![]()
![]()
![]()
![]()
![]() n CH2=CH-CH=CH2 + n CH2=CH [- CH2-CH=CH-CH2-CH-CH2- ]n
n CH2=CH-CH=CH2 + n CH2=CH [- CH2-CH=CH-CH2-CH-CH2- ]n
CN CN
Butadiene Acrylonitrile
The main features of addition (chain) polymerization are:
1. Only olefinic compounds can undergo addition polymerization.
2. No elimination of by products.
3. Double bond provides required bonding sites.
4. The addition of monomers takes place rapidly.
5. Linear polymers are produced.
6. The addition polymerization is brought about by free radical, ionic or co-ordination mechanism.
7. The molecular weight of the polymer is an integral multiple of the monomer.
8. The elemental composition of the polymer is same as that of monomer.
Condensation (step) polymerization:
A polymerization reaction in which bi or poly functional monomers undergo intermolecular condensation with continuous elimination of by products such as H2O, HCl, NH3 etc. is called condensation or step polymerization.
Egs: 1. Formation of Nylon66
n NH2-(CH2)6-NH2 + n HOOC- (CH2)4- COOH
![]() Hexamethylene diamine Adipic acid
Hexamethylene diamine Adipic acid
[-NH-(CH2)6-NH-CO-(CH2)4-CO-] n + 2n H2O
Nylon66
2. Formation of polyester
![]() nOH-(CH2)2-OH + n HOOC-C6H4-COOH [-O-(CH2)2-O-CO-C6H4-CO-] n
nOH-(CH2)2-OH + n HOOC-C6H4-COOH [-O-(CH2)2-O-CO-C6H4-CO-] n
Ethylene glycol Terephthalic acid Polyester +2nH2O
The main features of condensation polymerization are:
1. The monomers having two or more reactive functional groups can undergo condensation polymerization.
2. There is elimination of by products.
3. Polymerization proceeds through intermolecular condensation.
4. The polymer chain build up is slow and stepwise.
5. Polymerization is catalysed by acids or alkali.
6. Linear or cross-linked polymers are produced.
7. The elemental composition of the polymer is different from that of the monomers.
Mechanism of addition polymerization: Free radical mechanism:
The polymerization of ethylene monomer by free radical mechanism proceeds in three distinct stages: 1. Initiation 2. Propagation 3. Termination.
Initiation: It involves two reactions. The first is the production of free radicals usually by the homolytic dissociation of an initiator such as dibenzoyl peroxide to yield a pair of radicals. A free radical is an atomic or molecular species having an odd or unpaired electron. They are highly active species.
Heat heat .
![]()
![]() (C6H5COO)2 2C6H5COO. 2CO2 + 2C6H5 (R.)
(C6H5COO)2 2C6H5COO. 2CO2 + 2C6H5 (R.)
The second part of initiation involves the addition of this radical to the first monomer molecule to produce the chain initiating species.
.
![]() R. + CH2=CH2 R-CH2-CH2
R. + CH2=CH2 R-CH2-CH2
Propagation: In the propagation, the radical attacks another monomer to produce yet another free radical and the process continues until termination occurs.
. .
![]() R-CH2-CH2 + CH2=CH2 R-CH2-CH2-CH2-CH2
R-CH2-CH2 + CH2=CH2 R-CH2-CH2-CH2-CH2
![]()
n CH2=CH2
.
R-(CH2)n-CH2-CH2
In general
Termination: At some point, the propagating polymer chain stops growing and terminates. Termination is by 1. Coupling or combination i.e. a. the two growing chain may react with each other.
. .
R-(CH2)n-CH2-CH2 + CH2-CH2-(CH2)n -R
![]()
R-(CH2)n- CH2-CH2-CH2-CH2-(CH2)n-R
2. Coupling of growing chain with initiator free radical.
.
![]() R-(CH2)n-CH2-CH2 + R. R-(CH2)n-CH2-CH2-R
R-(CH2)n-CH2-CH2 + R. R-(CH2)n-CH2-CH2-R
3. Or by disproportionation in which a hydrogen atom of one radical center is transferred to another radical center. This results in the formation of two polymer molecules one saturated and another unsaturated.
. .
R-(CH2)n-CH2-CH2 + R-(CH2)n-CH2-CH2
![]()
Methods of polymerization: Industrial polymerization can be carried out by different techniques. The principal polymerization methods are
1. Bulk polymerization 2. Solution polymerization 3. Suspension polymerization 4. Emulsion polymerization.
Bulk polymerization: It is a homogeneous polymerization. The method is used for the liquid monomer. The monomer is taken in the liquid state and the initiator, chain transfer agents are dissolved in it. The reaction mass is heated or exposed to a radiation source for initiating the polymerization and is kept under agitation for proper mass and heat transfer.
Advantages:
1. Bulk polymerization is quite simple.
2. High purity products are obtained.
3. Products need neither isolation nor purification.
Disadvantages:
1. As the polymerization proceeds the viscosity of the medium increases leading to polymer products with broad molecular weight distribution.
2. Mixing becomes difficult.
3. Reaction is highly exothermic.
Applications:
This technique is used in the free radical polymerization of methyl methacrylate to poly- methyl methacrylate, styrene to polystyrene, vinyl chloride to PVC etc.
Solution polymerization: In this technique, monomer, initiator and chain transfer agents are dissolved in an inert solvent. The solvent helps to control viscosity increase and promote proper heat transfer.
Advantages :
Disadvantages:
Applications: This method is used for the production of polyethylene, PVC, polisobutylene, polyacrylonitrile etc.
Suspension (pearl) polymerization: This polymerization occurs in heterogeneous system. The water insoluble monomer is suspended in water as tiny droplets by continuous agitation. The droplets are prevented from coalescing by using small quantities of water- soluble polymers such as polyvinyl alcohol or colloids. The polymerization is made to occur in each droplet of the monomer using a catalyst. The reaction mass is heated ti initiate the polymerization. After the completion of polymerization pearl like polydispersed polymer mixture is obtained.
Advantages:
Disadvantages:
Application: This technique is used for the production of polyvinyl acetate, poly styrene, styrene-divinyl benzene etc.
Emulsion polymerization: In this method emulsion of water insoluble monomer and water is prepared and is stabilized by the addition of surface acting agents (surfactants) such as soap. Polymerization is initiated by the addition of water-soluble initiator such as potassium persulphate. After adding the initiator, the system is kept agitated in the absence of oxygen at 70oc.
Mechanism: The surfactant has hydrophilic head and hydrophobic tail. The water- soluble initiator links to the hydrophilic end whereas the monomer is linked to the hydrophobic end. At a little higher concentration it gets dispersed. When the concentration of surfactant exceeds critical micelle concentration (cmc), the soap molecule form micelle (aggregation of 50-100 molecules) oriented with tails inwards and head outwards. Now, an initiator molecule at the polar end diffuses into the micelle ti initiate the polymerization process. As the polymerization progresses, there will be depletion in the number of monomers within the micelle. They are replenished by the medium. This continues till the polymer formed is big enough to come out, the process is terminated by combination. The pure polymer is isolated from the emulsion by the addition of de-emulsifier.
Advantages:
Disadvantages: Polymer needs purification.
Application: This method is used for the production of PVC, PVA etc.
Glass transition temperature (Tg): is the temperature, below which a polymer is hard and above which it is soft and flexible is called the glass transition temperature. The hard, brittle state is known as the glassy state and soft flexible state as the rubbery state.
Parameters affecting Tg:
Egs: polyethylene has low Tg compared to that of nylon6,6.
Importance of Tg:
Structure property relationship of polymers:
The fundamental properties, which influence the structure property relationship are molecular mass, polarity, crystallinity, molecular cohesion, the nature of the polymeric chains and stereochemistry of the molecule.
Impact and tensile strength of polymers and molecular mass: Density, melt viscosity, impact and tensile strength are a few mechanical properties of a polymer. Tensile strength and impact strength increases with molecular mass up to a certain point and then become constant. The melt viscosity of the polymer initially shows a gradual increase with the molecular mass and steep increase at higher molecular masses. For polymer to be commercially useful it should have low melt viscosity, high tensile and impact strength.
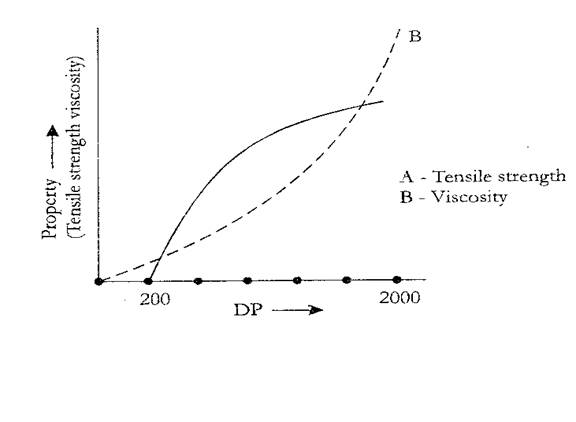
Crystallinity: Any polymer will contain a definite percentage of crystalline part and amorphous part. The degree of crystallinity depends on how best the polymer chain can be closely packed. Crystalline regions of a polymer are formed when their individual chains are linear (without branching), contain no bulky substituents and are closely arranged parallel to each other. The chains of polymer may be held together by vander wall’s force, hydrogen bonding or polar interaction. A polymer with high degree of crystallinity has high tensile strength, impact and wear resistance, high density and high fusion temperature, it has high Tg, and melt viscosity.
Crystallinity of a polymer also depends on the stereo regular arrangement. Polymers like HDPE, isotactic and syndiotactic polypropylene etc are highly crystalline. On the other hand atactic polypropylene, polystyrene, PVC which have their substituents in a random arrangement are less crystalline.
Elasticity: Elasticity of a polymer material is mainly because of the uncoiling and recoiling of the molecular chains on the application of force. For a polymer to show elasticity the individual chains should not break on prolonged stretching. Breaking takes place when the chains slip past each other and get separated. In rubber this is avoided by molecular engineering such as 1. Introducing cross- link at suitable molecular position 2.
Avoiding bulky side group such as aromatic and cyclic structure in the repeat unit 3. Introducing more non- polar groups in the chains so that the chains do not separate on stretching. The structure should be amorphous, this can be brought about by introducing plasticizer molecule in the polymer chain by co-polymerization or by7 compounding the rubber with a suitable plasticizer liquid.
Elastic deformation( rheology) of polymer: This can be studied by applying stress on the polymer material and finding the deformation caused. Polymers, since they contain both crystalline and amorphous regions, exhibit a complex behavior. The deformation depends upon on the degree of crystallinity, degree of cross- linking and the glass transition temperature.
Chemical resistivity: If a polymer is attacked by a reagent it undergoes softening, swelling and loses strength. Chemical resistivity of polymers depends on number of factors like presence of polar and non- polar groups, the molar mass, degree of crystallinity, extent of cross linking.
Polymers with non- polar groups undergo a welling and dissolution in non-polar solvents like benzene, toluene and carbon tetra chloride etc. Polar polymers containing –OH group or –COOH group are soluble in polar solvents like water, alcohol etc. Polymer containing ester group (polyester) undergo hydrolysis with strong alkalis at high temperature. Polyamide like nylon containing –NHCO- group, NHCOO group can be hydrolysed by using strong acids or alkali.
Polyalkenes, PVC, Flourocarbon are some polymers, which have high degree of chemical resistance. For a given polymer resistivity increase with increase in molar mass. Linear polymers have lower resistivity than branched chain and cross- linked polymers.
SOME COMMERCIAL POLYMERS:
POLYETHYLENE: is obtained by the polymerization of ethylene at high temperature.
![]() n (CH2=CH2) (-CH2-CH2-)n
n (CH2=CH2) (-CH2-CH2-)n
There are two commercial rate of polyethylene 1. LDPE (low density polyethylene) 2. HDPE (high density polyethylene)
LDPE: is obtained when ethylene is polymerized under very high pressure at 150-2500c in presence of oxygen or azocompounds.
150-2500c
![]()
![]() n (CH2=CH2) (-CH2-CH2-)n
n (CH2=CH2) (-CH2-CH2-)n
1500atmp.
Properties: It has a linear structure with extensive branch. It has low density i.e (0.91-0.92g/cc), low crystallinity, low softening point (110-1150c) and molecular weight is between 20,000-50,000.
APPLICATIONS: 1.It is used for packing food and textile materials.
2.It is used for moulding articles such as toys, tubes, pipes etc.
3. It has excellent electrical insulation properties. It is used for cables and wire coverings.
TEFLON (PTFE- polytetrafluoro ethylene):
Teflon is the trade name for PTFE. The monomer TFE is obtained by the following reaction.
![]() CHCl3 + 2HF CHClF2 + 2HCl
CHCl3 + 2HF CHClF2 + 2HCl
Chloroform Chlorodifluoro methane
![]() 2CHClF2 CF2=CF2 + 2HCl
2CHClF2 CF2=CF2 + 2HCl
TFE
Teflon is usually manufactured by emulsion polymerization of TFE using peroxide as initiators.
peroxide
![]() n CF2=CF2 (-CF2 – CF2 -)n
n CF2=CF2 (-CF2 – CF2 -)n
Teflon
Properties: 1. High degree of crystallinity 2. High m.p 3. High density 4. Insoluble in any solvent 5. High resistance to corrosive chemicals, oxidizing and reducing agents.6. Not wetted by either oil or water.7. Remains slippery over a wide range of temperature (-40 to 3000c). High thermal stability, good mechanical strength 9. Excellent electrical insulating properties.
Applications: 1. It is used for insulation of motors, generator, transformers, coils, capacitors, wires and cables.
2. Teflon is coated on articles such as bakery trays, frying pans and food processing equipments.
3.Gaskets, industrial filters, widely used in army weapons.
4.Teflon is an ideal lubricant therefore used for non- lubricated bearings and as a dry lubricant.
PMMA (poly methyl methaacrylate or plexi glass):
It is obtained by the polymerization of methylmethacrylate using hydrogen peroxide as initiator.
![]()
![]()
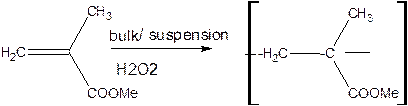
Methyl metheacrylate is obtained by the follow![]()
![]() ing method. Acetone with hydrogen cyanide gives acetone cyanohydrin. The hydrolysis of acetone cyanohydrin with sulphuric acid yields methylacrylic acid, which on estrification with methyl alcohol gives methyl methacrylate.
ing method. Acetone with hydrogen cyanide gives acetone cyanohydrin. The hydrolysis of acetone cyanohydrin with sulphuric acid yields methylacrylic acid, which on estrification with methyl alcohol gives methyl methacrylate.
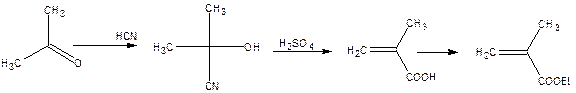
Plexiglass is a linear thermoplastic. It has excellent optical properties. It is highly resistant to heat, chemicals and water. It readily allows UV rays to pass through it. Hence it is used for making lenses, artificial eyes, light fixtures etc. It also finds uses as paints and adhesives. Air craft windows, instruments, dentures (set of artificial teeth).
Polyurethanes: contains –NHCOO- group and are formed by the reaction between diisocyanate and diol egs: Perlon-U is obtained by the reaction of 1,4-butane diol with 1,4-hexane diisocyanate.
n [ O=C=N-R-N=C=O ] + n [ OH-R’-OH ] à { -CO-NH-R-NH-CO-O-R’-O-}n
Properties:1.polyurethanes are less stable than polyamides (nylons) at elevated temperature (because of the presence of additional oxygen in the chain which increases its flexibility, the M.P of polyurethanes is much less than that of the corresponding polyamides.)
2.They are characterized by excellent resistance to abrasion and solvents.
Applications: 1. It is used for floor coating for gymnasium and dance floors where high abrasion resistance is required.2. Used as surface coatings, films, foams and adhesives.3. They are used for cushions because of improved strength, lower density and easier fabrication. 4. It is used in lightweight garments and swim suits because of its stretching property.5. They are used to cast to produce gaskets and seals.
Polycarbonates: are polymeric materials, which have repeat carbonate interunit linkages
(-o-coo-). Linear polycarbonates are formed by the condensation of Bisphenol-A with diphenyl carbonate.
Bisphenol-A is formed by the reaction between phenol and acetone.
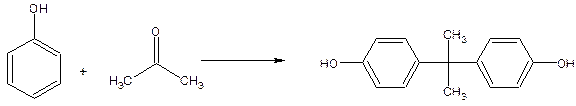
Diphenyl carbonate is obtained by the reaction between phenol and phosgene in an alkaline medium in the presence of a tertiary amine as catalyst.
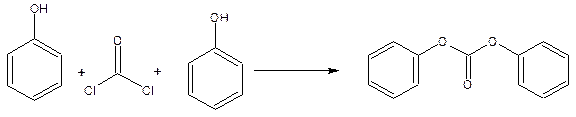
The condensation of Bisphenol-A with diphenyl carbonate at 2000c under reduced pressure gives polycarbonate.
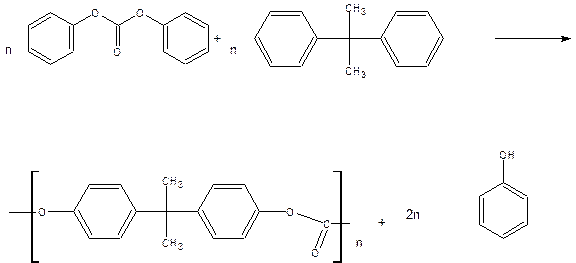
Properties: 1.High m.p 2. Tensile strength and impact resistance.3. It has excellent mechanical properties. 4. It is soluble in acids and alkali.
Uses: The polymer is used in the manufacture of safety goggles, telephone parts, automobile taillight lenses and unbreakable glazing appliances.
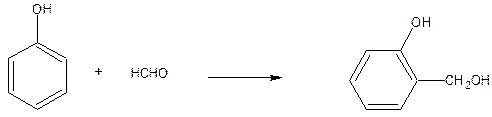
Phenol-formaldehyde resin (Bakelite) or Phenoplasts: Phenoplasts are prepared by the reaction between phenol and formaldehyde using basic or acidic catalysts.
Initial reaction results in the formation of mono, di, and trimethylol phenols depending on the ration of phenol and formaldehyde.
If the ratio is 1:1
![]()
If the ratio is 1:2 dimethylol phenol is formed

If the ratio is 1:3 trimethylol phenol is formed

Depending on the molar ratio of phenol, formaldehyde and catalyst two separate kinds of phenol resins are prepared they are novolac and resol.
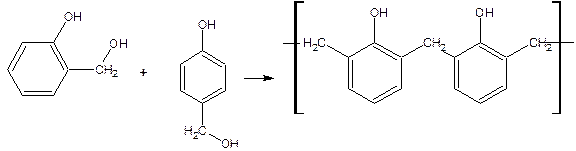
Novolac formation occurs between the ratio of phenol and formaldehyde is greater than unity and in the presence of acids Thus obtained monomethylol phenol then condensed with phenol form dihydroxy-diphenyl methane (novolac).Between two methylol group with the elimination of water ether linkage exists the resin obtained is called resol.
Further heating of novolac and resol in the presence of coumarin agents during moulding causes excessive cross linking in the formation of a hard rigid, insoluble polymer called phenol-formaldehyde or bakelite.
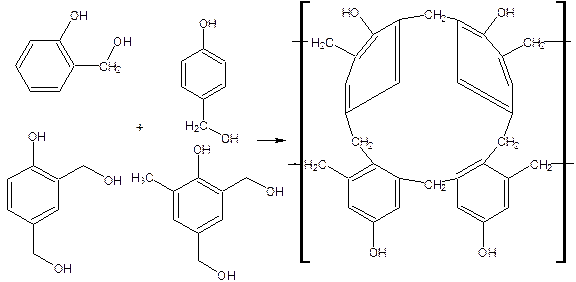
Properties: Phenolic resins are rigid, hard, scratched or abrasion resistance, water resistance except alkali because of the presence of free –OH group in the structure they posses excellent insulating character and good thermal resistance.
Uses: They are used for making electrical parts like switches, plugs, switch holes, heaters etc. They are also used for making moulding articles like telephone, television parts etc.
Elastomers: Rubber are high polymers which undergo elastic changes when subjected to an external force but readily regain their original position when external force is removed. Rubbers are therefore referred to as elastomers. A rubber band can be stretched to 4 to 10 times to its original length. Elastomers can be obtained from two processes, which may be natural or synthetic.
Natural rubber: Occurs in the inner bark of tropical plants like heavea brasiliensis, gauyule guttaparcha etc. I n the form of milky suspension called latex. Latex is a dispersion of isoprene during the treatment of latex these isoprene molecule polymerized to form long coil chain of polyisoprene.
Natural rubber is a linear high polymer of isoprene hence it may also called as poly isoprene.
Natural rubber has the following drawbacks (deficiencies):
Deficiencies of natural rubber and advantages of synthetic rubber.
Natural rubber |
Synthetic rubber |
Attacked by sunlight and air
|
Not attacked by air and sunlight (nitrile |
Less resistance to heat and cold. |
Greater resistance to heat and cold (nitrile |
Holds less air and water at high pressure. |
Hold more air and water even at high pressure (egs: Butyl rubber used in inner tubes of cars) |
Softens and swells on storing organic |
Don’t swell and can hold solvents better. |
Rubber property is lost at extreme Range of temperature. |
Rubber property is retained over wide temperatures. Egs: silicone rubber –900c to 3600c |
Ages quickly and loses surface luster |
Do not age easily (egs: polyurethane |
Buna-S (SBR, GRS) [styrene rubber or gov. regulated styrene rubber] :
Buna-S is the most important synthetic rubber it is obtained by the co-polymerization of 75% butadiene and 25% styrene in presence of peroxide catalyst at 50c.
Butadiene is obtained by the catalytic dehydrogenation of butane at 6000c.
Cr2O3 + Al2O3
![]() CH3-CH2-CH2-CH3 CH2=CH-CH=CH2 + 2H2
CH3-CH2-CH2-CH3 CH2=CH-CH=CH2 + 2H2
6000C
Styrene is obtained by the alkylation of benzene.
AlCl3
![]() C6H6 + CH2=CH2 C6H5-CH2-CH3
C6H6 + CH2=CH2 C6H5-CH2-CH3
![]() 6000C
6000C
Fe2O3/MgO dehydrogenation
C6H5-CH=CH2 +H2
![]()
![]() n CH2=CH-CH=CH2 + n C6H5-CH=CH2 [-CH2-CH=CH-CH2-CH-CH2-]n
n CH2=CH-CH=CH2 + n C6H5-CH=CH2 [-CH2-CH=CH-CH2-CH-CH2-]n
C6H5
SBR is a random co-polymer. It is vulcanized with sulphur. The vulcanized rubber is resistant to abrasion and high load bearing capacity. Mainly used for the manufacture of motor tyres, cycle tyres and tubes other uses of this elastomers are shoe soles, cable insulation, gaskets, tank lining, conveyor belts etc.
Butyl rubber: is manufactured by mixing isobutylene with 1.5 to 4.5% of isoprene. The monomers are polymerized in CH3Cl and anhydrous AlCl3 as catalyst at –950c.
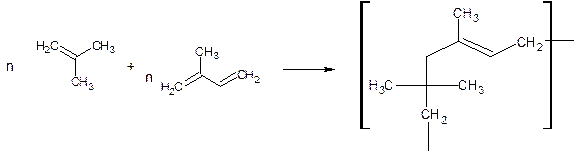
![]()
![]()
![]()
properties: Good chemical inertness, low gas permeability, less sensitive to oxidative aging, better ozone resistance and good solvent resistance.
Most of the butyl rubber are used in the manufacture of inner tubes for tyres.
ADHESIVES: They are mainly used to join a variety of substances such as metals, glasses, plastics, paper etc.
An adhesive is defined as a polymeric substance used to bind together two or more similar or dissimilar materials by surface attachment.
Adhesives join the surfaces in three distinct ways, they may keep the surfaces together by valence or intermolecular forces of attraction. They may fill the voids of porous or rough surfaces and hold the surfaces by interlocking action. The surfaces may partially dissolve in the adhesive.
The effectiveness and strength of an adhesive depends on various factors 1. The materials bonded.2. The solvent used and the effect of external conditions such as heat, light and environment. 3. It should not soften when used for binding plastics.
Adhesives are applied to surfaces by brushing, rolling, dusting or by trowel. Care must be taken to maintain a uniform thickness of the film. Adhesion is achieved by pressing together the materials to be bonded. To ensure good cohesion of adhesives the surfaces of materials to be joined must be cleaned before the adhesive is applied with paper and wood. Layers of grease, oil and lubricants are removed by means of suitable solvents. Loose dirt and oxide layers can be removed by brushing. The time required for an adhesive bonded joint to develop full strength varies from few second to few hours.
Types of adhesives: There are two types of adhesives 1. Natural adhesives :Egs, gum, glue, starch etc.
2. Synthetic adhesives: are low molecular weight polymers called resins.
egs, epoxy resins, phenol-formaldehyde, urea-formaldehyde etc. These resins have high strength, resistance to water and corrosion and are unaffected by weather.
Epoxy resins: [Araldite]: Epoxy resins are polymeric materials containing the epoxy group R-CH-CH-
![]()
![]() O
O
Epoxy resins are made by condensation polymerization of bisphenol-A and epichlorohydrin.
Bisphenol-A is made by reaction between phenol and acetone
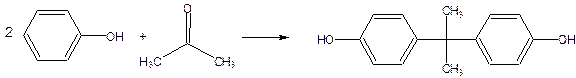
Epichlorohydrin is made from propylene by the following sequence of reaction
![]() CH2=CH-CH3 +Cl2 CH2=CH-CH2Cl +HCl
CH2=CH-CH3 +Cl2 CH2=CH-CH2Cl +HCl
![]()
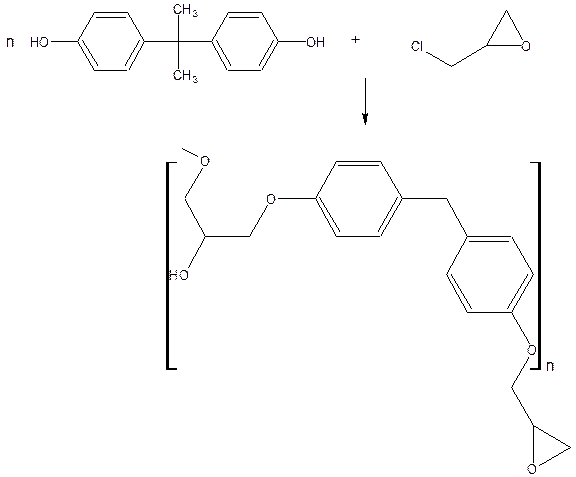
Properties: Epoxy resins have excellent adhesion to various surfaces, good abrasion resistance, excellent chemical resistance and electrical insulating properties.
USES: 1. They are used for laminating materials.
2.They are used to impart crease resistance and shrinkage resistance to fabrics.
3. They are widely used as structural adhesives.
4. They are also used in industrial flooring, highway surfacing and patching materials.
Chemical synthesis of polyaniline:
An aqueous solution of 0.1M (NH4)2S2O8 is added slowly with stirring to a 1.0M aniline hydrochloride dissolved in 1.0M aqueous solution of HCl. After the addition the mixture is stirred continuously for about two hours. Polymerization reaction is exothermic so to maintain the temperature 3-4oc, the reaction vessel is placed in an ice bath maintained at –1oc for one hour with constant stirring. During polymerization, color changes from light blue to blue green to copper tint to green. Polyaniline finally gets precipitated in the green form. This is filtered, washed with distilled water followed by washing with methanol or acetone until the washings are colorless. The residue is transferred to a beaker containing 1.0M aq. Solution of HCl and allowed to stand overnight. The precipitate is filtered and dried under vacuum at 60-80oc for 8 hours when a green salt, emaraldine is obtained. This is stirred with 0.1M NH4OH for 6 hours at a PH of 9 filtered, washed with distilled water followed by methanol and finally with diethylether. The resulting solid is dried at
60-80oc usnder vacuum.
Application
Polymer composites:
Two or more distinct components, which combine to form a new class of material suitable for structural applications are referred to as composite materials. A composite containing polymer matrix is known as polymer composite.
The properties of the composite system are not attainable by the individual components acting alone.
Properties of polymer composites:
Kevlar: Kevlar belongs to a family of aramids. It is a aromatic polyamide with the name poly [para-phenylene terephthamide]
The linkage through para position s of the phenyl rings gives Kevlar a strong ability to stretch and hence its extra strength.
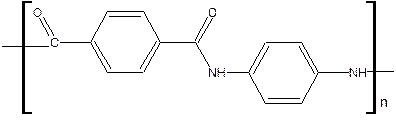
It has higher tensile strength and modulus than fiberglass. Kevlar fibers are used for structures requiring good stiffness, high abrasion resistance and lightweight.
Used in lightweight boat hulls, aircraft fuselage panels, pressure vessels, high performance racecars, bulletproof vests and puncture resistant bicycle tyres.
Carbon fiber: Carbon fiber is a polymer, which is a form of graphite with carbon ring structure.
Preparation of carbon fibre: is made by heating polyacrylonitrile. The polymer cyclises through the cyanogroups to form a polycyclic chain. The resulting solid is heated gradually. Then it is slowly roasted at 400-600oc when the adjacent chains join together losing more hydrogen gas. Then the temperature is gradually raised to 2000oc to get wider ribbon like mass.
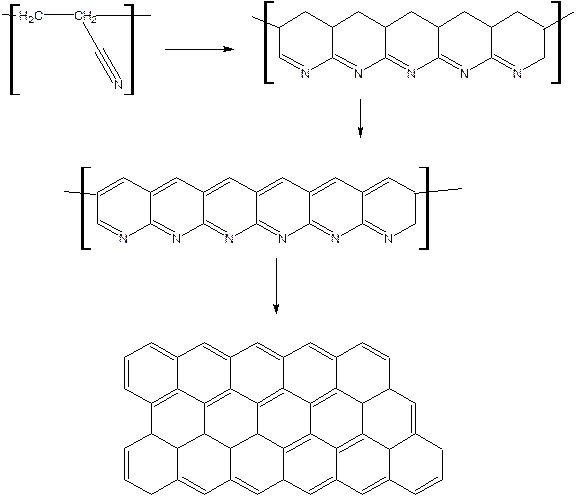
Carbon fiber reinforced composites are very strong and are often stronger than steel but lighter. They are used for making parts of aeroplanes and the space shuttle, tennis rackets and golf clubs, weaving machines, missiles, agricultural etc.
Applications of polymer composites:
It has higher tensile strength and modulus than fiberglass. Kevlar fibers are used for structures requiring good stiffness, high abrasion resistance and lightweight. Used in light weight boat hulls, air craft fuselage panels, pressure vessels, high performance race cars, bullet proof vests and puncture resistant bicycle tire.
Source: https://thevtu.files.wordpress.com/2009/01/highpolymers.doc
Web site to visit: https://thevtu.files.wordpress.com
Author of the text: indicated on the source document of the above text
If you are the author of the text above and you not agree to share your knowledge for teaching, research, scholarship (for fair use as indicated in the United States copyrigh low) please send us an e-mail and we will remove your text quickly. Fair use is a limitation and exception to the exclusive right granted by copyright law to the author of a creative work. In United States copyright law, fair use is a doctrine that permits limited use of copyrighted material without acquiring permission from the rights holders. Examples of fair use include commentary, search engines, criticism, news reporting, research, teaching, library archiving and scholarship. It provides for the legal, unlicensed citation or incorporation of copyrighted material in another author's work under a four-factor balancing test. (source: http://en.wikipedia.org/wiki/Fair_use)
The information of medicine and health contained in the site are of a general nature and purpose which is purely informative and for this reason may not replace in any case, the council of a doctor or a qualified entity legally to the profession.
The texts are the property of their respective authors and we thank them for giving us the opportunity to share for free to students, teachers and users of the Web their texts will used only for illustrative educational and scientific purposes only.
All the information in our site are given for nonprofit educational purposes