Guideline for hygienic assessment of elastomers in contact with drinking water (Elastomer Guideline)
Draft: 10 August 2011
1. Preliminary remark
This Guideline can be used to assess elastomers in contact with drinking water within the meaning of the Drinking Water Ordinance, Section 17 (1). It replaces the KTW Recommendation part 1.3.13 "Rubber made from natural and synthetic rubbers".
This Guideline shall not apply to thermoplastic elastomers (TPE) nor to silicones. Silicones can be assessed according to the requirements of the KTW Guideline.
This Guideline was developed by the Federal Environment Agency (Umweltbundesamt - UBA) in cooperation with the KTW-AG (Joint Working Group of the Drinking Water Commission of the Federal Environment Agency and the Committee for Consumer Products of the Federal Institute for Risk Assessment on the hygienic assessment of plastics and other non-metal materials in contact with drinking water) and the trade association of the German Kautschukindustrie e.V. (wdk).
Like the other Guidelines of the Federal Environment Agency on the hygienic assessment of organic materials in contact with drinking water (KTW, Coating and Lubricants Guideline), this Guideline is broken down into three parts,
This Guideline is not a legal norm and is therefore non-binding. It represents the current state of science and technology concerning the sanitary requirements for elastomers in contact with drinking water within the meaning of the Drinking Water Ordinance 2001 (TrinkwV 2001), last amended on 3 May 2011 (Federal Law Gazette 21, p. 748-774).
Article 17 (1) of TrinkwV 2001 states that for the new construction or maintenance of installations for extracting, processing or distributing water for human consumption, “only those materials [may] be used which when in contact with water, do not release substances in concentrations which exceed limits deemed unavoidable according to generally acknowledged technical standards. Furthermore, materials which directly or indirectly diminish the level of protection of human health provided for by this Ordinance, or which alter the odour or flavour of the water;...”.
It can therefore be assumed that elastomers in contact with drinking water which comply with the requirements of this Guideline will also satisfy the sanitary requirements of TrinkwV 2001.
To prove the hygienic safety of the respective elastomers with respect to microbiological requirements, in addition to and independently of this Guideline, the materials used must pass a test in accordance with DVGW-Arbeitsblatt W 270.
The compliance of a product with an element from elastomers with generally accepted rules of the Art and the requirements of TrinkwV 2001 can be recognised by the certification symbol of an industry certification body, e.g. DVGW Cert GmbH.
Elastomers (hard and soft rubbers) are high polymers, organic networks, which are able to resist and reverse large deformations.
2.1. Definition of elastomers
Elastomers are multi-compound systems and consist of the main components explained below:
Rubber is the designation for non-cross-linked polymers, which can be cross-linked (vulcanised), with rubber elastic properties at 20°C. Rubbers are systematically broken down into natural and synthetic rubbers. Natural rubber consists almost exclusively of saps (latex). Synthetic rubbers are artificially manufactured polymers, which are obtained by polymerising monomers. According to the many different areas of application and the requirements for thermal and chemical stability, there is a variety of synthetic rubber types. The material properties can be carried widely in terms of their limits through the copolymerisation of various monomers.
Fillers, e.g. soot or fine silicic acid, have an strengthening effect on the polymer matrix and are used to increase the breaking strength and abrasive resistance of the product, for example.
Plasticisers are added to the rubber compound, for example, to adjust the hardness of the vulcanisate or to improve flexibility in the cold.
Anti-ageing agents protect the elastomers against external effects. For example, they counteract the harmful effects of oxidation, heat, light or even the ozone on the elastomer.
Processing aids have a wide range of uses in a rubber compound. These include, inter alia, improving the deformation resistance of rubber blanks, increasing workability during the mixing process and/or during forming, and many more.
Cross-linking agents such as sulphur, sulphur donors peroxides allow the rubber compound to be vulcanised into elastomer. Accelerating and retarding agents are also used for vulcanisation with sulphur.
The composition and the manufacturing processes determine the final properties of the elastomers. The construction of the compound and the manufacturing process are important processes that require a wide range of machines and require a large amount of energy. In most cases manufacturing is carried out in three stages. This is shown in figure 1:
Fillers, plasticisers, processing aids, anti-ageing and cross-linking agents
Mixing process Forming and vulcanisation
Elastomers
Rubber compound
Rubber
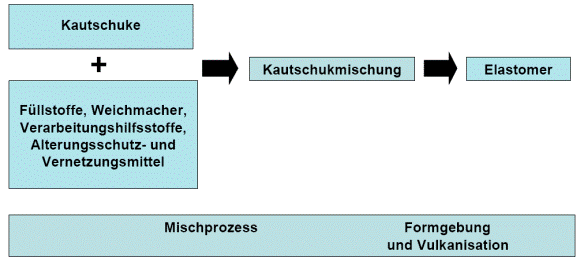
The individual components listed in 2.1 are combined on a rolling mill or in an internal mixer with the addition of energy to produce the non-cross-linked rubber compound.
The rubber compound can be formed into rubber blanks in a variety of ways. One of the simplest methods is extrusion. This involves pressing the rubber compound through shaped nozzles to form flat strips, round cords, profiles or hoses depending on the shape of the nozzle. Calendars are used to manufacture films, plates or rubberised fabrics. Calendars consist of more than two temperature-controlled mills.
Vulcanisation cross-links the rubber compound or the rubber blank three-dimensionally with the addition of cross-linking agents and heat. This generally creates highly elastic materials, also known as elastomers.
The most widespread vulcanisation process is press heating. In the traditional type of pressing, a prepared, roughly preformed compound blank is placed into a preheated metal mould, which is then sealed and placed between the plates of a heated press. This softens the rubber compound, adopts the shape of the cavity under pressure and is then fully vulcanised.
A more recent development, which is specifically designed for the mass production of moulded parts, is injection moulding. This involves automatically pressing the hot rubber compound into the cavities in the mould.
For other articles, (e.g. products that are coated with elastomers), vulcanisation is carried out in autoclaves or in vulcanising autoclaves, which work on the principle of a pressure cooker.
For elastomers that are manufactured in continuous lengths, e.g. profiles, hoses, conveyor belts, cables, etc. special equipment is used to allow continuous vulcanisation. This can be carried out, for example, in a liquid bath, in a hot-air chamber or in a steam chamber.
Elastomers are used in the drinking water supply for a wide variety of applications. A summary of these can be found in Annex 5.
Only the starting substances used in parts 1 + 2 of the positive list (Annex 1) should be used to manufacture the elastomers assessed under this Guideline (see 5). The use of substances under the De Minimis Guideline is also possible.
The positive list is broken down into three parts:
Part 1 of the positive list contains substances that are assessed toxicologically. The assessments were carried out by the European Food Safety Authority (EFSA), formerly the Scientific Committee on Food (SCF), or in close cooperation with the Federal Institute for Risk Assessment (BfR).
Part 2 of the positive list contains part-assessed substances, the use of which is accepted until December 2016. For inclusion in Part 2 and the relevant temporary applications of these substances, at least one safety assessment is required prior to a complete toxicological assessment. This involves presenting data on the migration of the substance in question and, if necessary, on its reaction and decomposition products. This information is necessary in order to assess potential exposure. The transitional rule can only be applied to substances that would normally be used to manufacture elastomers in contact with drinking water.
Part 3 contains examples of rubbers that are normally used in the manufacture of elastomers. The rubbers are listed along with their DIN ISO 1629 codes. To achieve certain product properties, offcuts of rubber polymers are used with each other or with polymers that meet the KTW Guideline. The starting substances for rubbers listed (preparations) must be listed in Parts 1 or 2 of the positive list.
The starting substances for the manufacture of elastomers must be of good technical quality and purity. The rubbers must be produced in accordance with good manufacturing practice.
For Part 1 and Part 2 of the positive list the following applies:
In this positive list the "Monomers for rubbers" and "Cross-linking agents" must satisfy the "Monomers and other starting substances" of Regulation (EU) No 10/2011 .
Cross-linking agents are broken down into peroxides and their co-agents, carbamates, thiocarbamates, thiurames, sulfonamides, guanidines, xantogenates, thiophosphates, mercapto accelerators and other accelerators..
The positive list also contains other formulation components fillers, plasticisers, anti-ageing agents, processing aids and colourants.
The use of biocide additives in drinking water materials to achieve a biocidal effect on the resulting manufactured products (biocide equipment, biocide additive) is rejected by UBA. In aqueous preparations (aqueous starting substances and intermediate products such as latex dispersions), however, it may be necessary, to use biocide additives to maintain the stability of a preparation of microbiologically biodegradable substances until used (in-can preservation). These in-can preservation agents may be contained in low-level concentrations in the preparation and are no longer active in the final product due to other elements in the formulation. The in-can preservation agents must be included in the positive list (Annex 1) and must be stated in the formulation review.
The positive list is set out in table format. Column 1 shows the "EEC Packaging Material Reference Number (Ref. No) from Regulation (EU) No 10/2011. Column 2 contains the CAS (Chemical Abstracts Service) number. Column 3 contains the substance name.
Column 4 shows the DWPLL values for several substances that are used as test criteria in the migration test (see 5.4).
The DWPLL (Drinking Water Positive List Limit) is a human-toxicologically derived temporary drinking water limit for material-specific substances and is used to quantify a substance migration to be assessed as acceptable in the text system at the point in time determined in the Guideline.
A DWPLL value corresponds to 10% of the substance-specific Tolerable Daily Intake (TDI) of a 60-kg person in 2 litres of drinking water.
The DWPLL may also have been calculated using the Specific Migration Level (SML) of Regulation (EU) No 10/2011 with the formula DWPLL = 1/20 SML of the Federal Environment Agency (UBA), or derived by UBA in cooperation with the Federal Institute for Risk Assessment (BfR) according to the principles of the EFSA.
The designation "TOC" in column 4 implies that the substance cannot be specifically determined, but is instead covered by the basic requirement for the TOC parameter.
Column 5 shows the "QM" limit for the residual content in the vulcanised elastomer, "QMA" contains a residual content determination of the vulcanised elastomer, which is based on a surface area of 6 dm2 (area-based residual content). These requirements have been adopted from Regulation (EU) No 10/2011. If the substance in the test water can be determined, it is possible, assuming that 1 kg of food is packaged in a cube with a surface area of 6 dm², that a SML value can be derived from the QMA value and the DWPLL value can be determined.
Column 5 also contains in some cases the purity requirements for the substance input listed.
The addition of a substance to part 1 of the positive list is only permitted on application by a manufacturer (applicant) to the Federal Environment Agency. The positive list is updated approximately once per year.
The application shall be subject to the requirements of the EFSA questionnaire (“Note for guidance” http://www.efsa.europa.eu/en/efsajournal/pub/21r.htm that is contained in Chapter III of the European Community’s questionnaire), and which is divided into sections 1 to 8.
Section 8 of the questionnaire describes the requirements for the toxicological data to be submitted, whose extent is determined by the level of migration of the requested substance in deionised water. All existing toxicological data must be presented.
When applying for substances that have already undergone toxicological assessment (e.g. by the EFSA) only the requirements of points 1 to 4 must be met.
No new substances are being added to part 2 of the positive list.
Elastomers in contact with drinking water must be fit for purpose. The requirements of the technical standard apply regardless of this Guideline.
The assessment under this Guideline is carried out for a product that is manufactured from a vulcanised elastomer.
All of the substances used to manufacture the rubber compounds and the rubber itself must be toxicologically assessed and listed in the positive list according to their technological function (Annex 1 Parts 1 and 2). When using pre-cross-linked or plugged rubber, the additives in the starting substances must be taken into account in relation to the DWPLL values stated.
For certain substances that are not included in the positive list of the Elastomer Guideline, the De Minimis Guideline can be applied if the requirements stipulated therein are met.
The substances used when manufacturing elastomers in contact with drinking water must be of a technical quality and purity that is fit for the planned and proposed purpose of the elastomers.
The test under 6.4 indicates that the test values of the basic (5.1) and additional requirements (5.2) and the formulation requirements for individual substances (5.3) are observed in the migration water samples.
The external characteristics of odour, flavour, clarity, colour and foaming of the migration water may not be changed.
For the cold water test, the odour and flavour thresholds (threshold odour number - TON, threshold flavour number – TFN) apply:
TON and TNF < 2 For the third migration period
For the hot water test the following applies:
TON and TNF < 4 For the seventh migration period
For the release of organic substances, measured as total organic carbon TOC), the following applies to the cold water test:
DWPLLTOC = 0.5 mg/l for the third migration period
For the hot water test the following applies:
DWPLLTOC = 0.5 mg/l for the seventh migration period
The TOC is defined as a non-volatile organic carbon (NPOC) in accordance with DIN EN 1484.
The additional requirements laid down in table 1 shall apply.
The migration of the substances and substance groups listed in the table must be tested in accordance with 6.4 and checked against the DWPLL values quoted (see 5.3).
Depending on the type of cross-linking (sulphur cross-linking or peroxide cross-linking), tests are carried out either on mercaptobenzothiazole and N-nitrosamine, if N-nitrosamine formers are included in the formulation, or on peroxides using the methods specified in table 1.
Table 1: Additional requirements for elastomers
Substances/substance groups |
DWPLL in µg/l |
Test method* |
Zinc |
3000 |
DEV |
Formaldehyde |
150 |
50th Notification |
Primary aromatic |
N. N. (NWG = 2 µg/l) |
Specific proof with GC-ECD/GC-MS with derivatisation |
Table 1: Continued
Substances/substance groups |
DWPLL in µg/l |
Test method* |
|
Secondary amines |
250 µg/l (Total concentrations of tested amines) |
Specific proof as for PAA |
|
Types of cross-linking: |
|||
|
|
|
|
2-mercaptobenzothiazole |
400 µg/kg elastomer |
EN 1400-3: 2002 |
|
N-nitrosamine according to |
0.3 |
53rd Notification (Federal Health Gazette 37(1994)232), |
|
|
|
|
|
Peroxides |
No peroxide on the surface of the product |
Method to be announced |
|
All substances with a limit in column 4 of the positive list, which may be contained in the product, must be tested in terms of their migration according to 6.4. The concentration determined in the test is used to calculate the maximum concentration cTap(see 5.4) expected at the tap.
Instead of an experimental test, the migration can also be estimated using the Modelling Guideline (see 5.5).
For the cold water test the following shall apply:
cTap ≤ DWPLL for the third migration period
For the hot water test the following shall apply:
cTap ≤ DWPLL for the seventh migration period
In addition, the measured concentrations must not show a rising trend.
For substances with the indication “TOC” in column 4 of the positive list, the requirement for the individual substance is observed if the basic requirements are met.
For substances with the indication "QM" or "QMA" in column 5, a review of the residual content of the substance in the vulcanised elastomer is required. The QM and QMA limits apply independently of the elastomer product group. If the substance in the test water can be determined, it is possible, assuming that 1 kg of food is packaged in a cube with a surface area of 6 dm², a SML value can be derived from the QMA value and the DWPLL value can be determined for testing in place of the QMA value.
Compliance with the purity requirements of the substances used can be confirmed by a Declaration of Conformity by the supplier.
The maximum expected tap concentrations (cTap) differ for the various product groups according to conversion factors stated in table 2 FC:
![]()
Where
FC : Conversion factor according to table 2
cmeasured: Concentration measured in the migration test according to DIN EN 12873-1
S/V: Surface-to-volume ratio according to DIN EN 12873-1
t: Duration of the migration period according to DIN EN 12873-1
Table 2 lists the product groups of pipes, tanks and fittings, where the requirements are further differentiated according to their place of use within the water distribution system. The product group of seals is assigned the corresponding pipe dimensions.
Table 2: Product groups with the corresponding conversion factors
Product group |
Conversion factor |
Pipes with DN < 80 mm (domestic installation) |
20 |
Pipes of diameter 80 mm ≤ DN < 300 mm (supply pipes) |
10 |
Pipes of diameter DN ≥ 300 mm (main pipes) |
5 |
Fittings for pipes with DN < 80 mm |
4 |
Fittings for pipes with 80 mm ≤ DN < 300 mm |
2 |
Fittings for pipes with DN ≥ 300 mm |
1 |
Fittings for pipes with DN < 80 mm |
0.4 |
Seals for pipes with 80 mm ≤ DN < 300 mm |
0.2 |
Seals for pipes with DN ≥ 300 mm |
0.1 |
Tanks in domestic installations |
4 |
Tanks outside domestic installations |
1 |
Repair systems for tanks in domestic installations |
0.04 |
Repair systems for tanks outside domestic installations |
0.01 |
In Annex 5 to the Elastomer Guideline, typical elastomer products are assigned to the product groups stated in table 2.
In place of the experimental test, the migration can also be estimated using the Modelling Guideline (see 5.5), insofar as the applicability of generally recognised, scientifically proven diffusion models and characteristics have been determined.
The Practical Guide (Annex 1) contains specific parameters for the most important organic materials.
The report by C. Simoneau, et al. (2010) is also available.
In the case of other organic materials used in contact with drinking water, these parameters must be determined specifically for each material or product before modelling can be applied. The tests required for this are also described in the Practical Guide (Annex 1).
Generally accepted technical standards include, for instance, the Technical Rules of the German Technical Association for Gas and Water (DVGW).
Commission Regulation (EC) No 10/2011 of 14 January 2011 on plastic materials and articles intended to come into contact with food: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2011:012:0001:01:EN:HTML
German standard methods for the examination of water, waste water and sludge (DEV)
The following PAA must be tested: Aniline, o-toluidine
Not detectable
The corresponding DWPLL values for the respective amine (see Annex 1 of the positive list in this Guideline) must be defined independently of this additional requirement.
Test method: Pietsch et al (1996) Fresenius j. Anal. Chem. 355:164-173 or Pietsch et. al. (1997) Vom Wasser 88: 119-135
The following secondary amines must be tested: Dibutylamine, diethylamine, dimethylamine, dicyclohexylamine, cyclohexylethylamine, diphenylamine, dibenzylamine, benzyl-N-methylamine, benzilidenbenzylamine, N-methylaniline, N-ethylaniline, N-butylaniline
The corresponding DWPLL values for the respective amine (see Annex 1 of the positive list in this Guideline) must be defined independently of this additional requirement.
The N-nitrosamine formers are identified in the positive list with an appropriate footnote "N" (zinc-N-dibutyldithiocarbamate, dimethyldiphenylthiuramdisulfide, tetraethylthiuramdisulfide, tetramethylthiuramdisulfide). The N-nitrosamine must be determined according to TRGS 552.
Testing of foodstuffs; determination of nitrosamines in foodstuffs
Guideline for the mathematical estimation of the migration of individual substances made of organic materials into the drinking water
Diameter
Guideline for the mathematical estimation of the migration of individual substances made of organic materials into drinking water
Practical Guide has been withdrawn by the EU Commission. Annex 1, Mathematical Models, can however still be downloaded from
http://ihcp.jrc.ec.europa.eu/our_labs/eurl_food_c_m/files/PRACTICAL%20GUIDE%20_2003.04.15__annex%201%20modelling.pdf/view
"Applicability of generally recognised diffusion models for the estimation of specific migration in support of EU Directive 2002/72/EC" under http://publications.jrc.ec.europa.eu/repository/handle/111111111/14935
A prerequisite for the modelling is the determination of the amount of the relevant substance in the product tested (cP,0).
The method of analysis for determining cP,0 for the polymer must be presented by the raw material supplier, if there is no validated method available from the "Community Reference Laboratory for Food Contact Materials" (http://crl-fcm.jrc.it/index.php?option=com_methods&Itemid=80) or a DIN standard. Alternatively cP,0 can be used from the required quantity, if cP,0 does not change during the manufacture and/or processing of the product.
The modelling must satisfy the respective test conditions (test temperature and test cycle) of this Guideline (see 6.4). The concentration profile for the previous test period is used to calculate the migration for the following test period. The Modelling Guideline describes in detail modelling with the flow chart for the inclusion of modelling for the hygienic assessment of products in the framework of the Guideline.
Validated software must be used for modelling. The requirements for the software solutions to be used are detailed in the modelling Guideline.
If a product does not meet the requirements of the Guideline with regards to the individual substances to be tested after modelling the migration, proof can still be provided by way of an experimental test. The results of experimental tests must be weighted higher than those of the modelling.
In order to receive a test certificate for elastomers in contact with drinking water, the applicant must provide the test laboratory with the formulation components of the elastomer (indication of all components with the percentage by weight, the CAS No and material group according to the positive list for elastomer materials (formulation declaration in Annex 2).
The formulation information according to Annex 2 can be given separately by the elastomer manufacturer and the manufacturer of the preparations, if the exact designation of the respective products clearly indicates their unique assignment to the elastomer.
This clarifies the extent of the DWPLL values to be tested and the residual contents (QM QMA) for individual substances in the finished elastomer and the purity requirements for the listed substances or substance groups.
Furthermore, the proposed product group (according to 5.4, table 2) of the elastomer must be stated. In the case of multi-layer structured products, the formulations of all layers must be provided (e.g. for multi-layer hoses). In layers made from different materials, the corresponding Guidelines must be applied.
The test under this Guideline must be carried out by a test laboratory accredited in accordance with ISO/IEC 17025 or a test laboratory recognised by an accredited industry certification body.
6.3.1 Elastomers
The test should in principle be carried out on the elastomer products
Single-layer hoses are tested by filling.
For product groups with the same formulation (see table 2), which are manufactured using the same process, the test laboratory can select a mixed sample for testing (e.g. O-rings of one size group with different diameters).
If it is not possible to test the finished product, testing can be carried out on test plates with dimensions approx. 200 mm x 200 mm x 2 mm for single-layer elastomers. The test plates must have the same formulation and be vulcanised under the same temperature and time constraints as the products (Annex 4 of the Guideline).
6.3.2 Multi-layer products
Multi-layer materials, consisting of ply, layers or deposits of individual substances, the individual components of which have an influence on the surface in contact with water by diffusion, are tested as multi-layer products or multi-layer product components in consultation with the test laboratory
Multi-layer hoses are tested by filling.
Testing must be carried out in accordance with the standards DIN EN 1420-1: 1999, DIN EN 12873-1: 2004 and DIN EN 12873-2: 2005. Annex 3 contains the test conditions in abridged form. The test method and test results must be carefully recorded (Annex III of the test report). The test laboratory should examine whether the basic requirements, additional requirements and recipe-dependent requirements for individual substances for the proposed product group have been fulfilled.
In the migration test at (23 ± 2) °C and the odour/flavour test at (23 ± 2) °C, the test water samples from the first three test periods should be examined.
In the migration test and the odour/flavour test at higher temperatures, the test water samples from the first, sixth and seventh migration periods should be examined. The TOC parameter, however, should be determined in the first, second, third, sixth and seventh migration periods.
If the maximum expected tap concentration (cTap) of the third (cold water) or seventh (hot water) migration period observes the specific migration limit (SML value) of Regulation (EU) No 10/2011 defined in the calculation for the substance, but the calculated DWPLL value is exceeded, a fixed-term 5-year test certificate can be issued (without possibility of extension).
Standardised analytic procedures should normally be followed in testing the migration samples. Where no suitable analytic method currently exists for a particular substance, an analytic method of suitable accuracy, which enables an assessment of the recorded concentration to be made, may be applied until a standardised method is developed. Any hitherto unavailable analytic methods for substances in list 1 of the Positive List (list of assessed substances in Annex 1) should be developed by the manufacturer and test laboratories and notified to the Federal Environmental Agency. The test laboratory should entered the analytic procedures applied in table 5 in Annex 3 to the Guideline.
The complete test results must be inserted in the tables according to tables 4 and 5 of Annex 3 and appended as Annex I to the report. Compliance with the formulation requirements for individual substances (DWPLL values), which are subject to confidentiality, are recorded by the test laboratory with the number of substances and the note "Test value observed".
Instead of analytical proof of compliance with the DWPLL values, mathematical methods may be used to estimate the migration rate of individual substances from the elastomer into the drinking water. If modelling is used, appropriate documentation must be presented (see 5.5).
If the test is passed, a test report is to be prepared by the test laboratory which should include the information specified in tables 4 and 5 of Annex 3. This consists of the test certificate and the following annexes:
Annex I: Table with the full test results (see Annex 3 of the Guideline),
Appendix II: Formulation declaration (Annex 2 of the Guideline, completed and signed by the manufacturer/applicant and the test laboratory),
Annex III: Record of the testing procedure followed (cf. 6.4),
Annex IV: Selection and indicators for the test methods used.
The test certificate must contain the closing paragraph:
" The product ... (precise designation) has been tested in accordance with the Guideline on the hygienic assessment of elastomers in contact with drinking water by the Federal Environment Agency and has passed the test for the proposed product group(s) ..... in the temperature range up to ... °C.
Test certificates issued in accordance with this Guideline are valid for a period of 5 years.
For products manufactured using substances from part 2 of the positive list, the test certificate shall cease to the valid at the latest on 31 December 2016.
Test certificates for products from the same manufacturer which are produced in accordance with this Guideline may, if they comply with all the requirements listed in 6.4 in the initial test, be extended for 5 years without further experimental testing, providing that there has been no change in their formulation, in the relevant substance assessments (restrictions in the Positive Lists) or in the manufacturing process. Prior to extending the test certificate, the test laboratory must check that the formulation, the manufacturing process and the underlying positive list have not changed
On the test certificate it must be clearly noted if it was issued by way of an exception (use of limited substances, exceedance of DWPLL values) and therefore cannot be extended.
The test centres accredited for tests on organic materials in contact with drinking water report to the Federal Environment Agency once a year on the applicability of this Guideline.
As part of this, the following information must be reported in anonymous format pursuant to Annex 6:
Based on the feedback, the Federal Environment Agency will decide whether or not changes and/or additions to this Guideline are necessary.
Annex 1 to the Elastomer Guideline
Positive list for elastomers in contact with drinking water
The following table contains:
Column 1: the EEC packaging material reference number
Column 2: the Chemical Abstracts Service (CAS No) registry number
Column 3: the substance name
Column 4: the "human-toxicologically derived temporary drinking water limit for material-specific substances": Drinking Water Positive List Limit (DWPLL) in µg/l
Column 5: Residual content of QM in mg/kg polymer or QMA values in mg/6dm² or other requirements on purity/composition
For substances whose migration restriction is limited as a group, an appropriate number will be inserted in column 4.
N-nitrosamine formers are marked in the positive list in column 4 with an "N". For these substances the additional requirement "N-nitrosamine" must be tested.
For some substances, both a DWPLL as well as a QM or QMA value must be indicated for a restriction. In these cases, only one restriction has to be tested. Preference should be given to checking the DWPLL value.
The positive list also contains substances (acids, alcohols and phenols) that can occur in the form of salts. Since the salts in the stomach acids are normally converted into salts, alcohols or phenols, it is possible to use salts of lists acids, alcohols or phenols. This means that the salts (including double salts and acid salts) of aluminium, ammonium, barium, calcium, cobalt, copper, iron, lithium, magnesium, manganese, potassium, sodium and zinc of the listed acids, phenols or alcohols are included. For the aforementioned cations, the migration restriction is based on the limit values of Annexes 2 and 3 of TrinkwV 2011.
For all of the fillers used, the purity requirements of the BfR Recommendation LII must be met.
For all of the colourants used, the purity requirements of the BfR Recommendation IX must be met.
For the assessment of the aids to polymerisation for manufacturing rubber, the corresponding BfR Recommendation may be used.
Source: http://ec.europa.eu/growth/tools-databases/tris/nl/index.cfm/search/?trisaction=search.detail&year=2011&num=467&dLang=EN
Web site to visit: http://ec.europa.eu/
Author of the text: indicated on the source document of the above text
If you are the author of the text above and you not agree to share your knowledge for teaching, research, scholarship (for fair use as indicated in the United States copyrigh low) please send us an e-mail and we will remove your text quickly. Fair use is a limitation and exception to the exclusive right granted by copyright law to the author of a creative work. In United States copyright law, fair use is a doctrine that permits limited use of copyrighted material without acquiring permission from the rights holders. Examples of fair use include commentary, search engines, criticism, news reporting, research, teaching, library archiving and scholarship. It provides for the legal, unlicensed citation or incorporation of copyrighted material in another author's work under a four-factor balancing test. (source: http://en.wikipedia.org/wiki/Fair_use)
The information of medicine and health contained in the site are of a general nature and purpose which is purely informative and for this reason may not replace in any case, the council of a doctor or a qualified entity legally to the profession.
The texts are the property of their respective authors and we thank them for giving us the opportunity to share for free to students, teachers and users of the Web their texts will used only for illustrative educational and scientific purposes only.
All the information in our site are given for nonprofit educational purposes