Intergranular corrosion is a specific location corrosion type in that only the areas on or adjacent to grain boundaries are dissolved. No corrosion is found in areas away from the grain boundaries. One of the classic examples of intergranular corrosion is "weld decay" which will be dealt with later in this section. Another type of intergranular corrosion is “exfoliation” in which the top layers of aluminum alloys essentially delaminate from the surface due to intergranular corrosion. In this type of corrosion, the metal is taken apart rather like taking a jigsaw puzzle to pieces one part at a time.
The reasons that grain boundaries and the areas adjacent to them become active corrosion site can depend on many factors. In Al-Mg alloys, the magnesium tends to segregate or preferentially collect at the grain boundaries. As magnesium is a very active element, then the grain boundaries become active and dissolve. The grain surface area that is not being attacked become cathodic with a resulting large cathode to small anode ratio which increases the corrosion rate for the grain boundary regions. Other mechanisms involve grain boundary phases that act either as anodes or cathodes to the remainder of the grain. Aluminum alloys are very prone to this type of attack.
Weld Decay or Sensitized Stainless Steels.
304 and 316 austenitic stainless steels are face center cubic structure with no second phases. A 304 stainless steel has 0.06 to 0.08 wt%C, 16-18 wt% Cr and 8-10% Ni with the balance of the composition being iron. It obtains its corrosion resistance from the passivation effects of the chromium, and the single phase structure helps. If either of these factors are compromised then the corrosion resistance decreases.

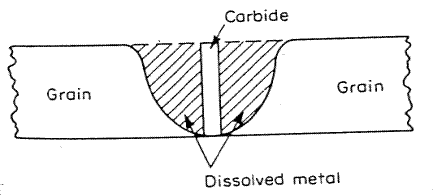
In the figure above, corrosion occurred on either side of the weld on a stainless steel tank. These tanks were prone to weld decay.
One way to decrease corrosion resistance is to “sensitize” the stainless steel, which is when the complete stainless steel part undergoes intergranular corrosion. When this happens during the welding process, and it is localized to an area adjacent to the weld and it is called “weld decay”. The basic mechanism is the same in either case.
The combination of chromium and carbon in the stainless steel provide the necessary ingredients to form chromium carbide, of atomic composition Cr23C6, or 23 atoms of chromium for every 6 atoms of carbon. The other necessary ingredient is thermal treatment. The carbide is stable between the ranges of 950 and 1450oF, but requires some time to grow due to the nucleation and growth required for second phases. The nucleation site of the chromium carbide is the austenite grain boundaries. The second phase therefore grows on the grain boundaries. The distribution of the chromium in the steel then becomes important. Chromium can only diffuse by substitutional diffusion in the matrix which is slow or along grain boundaries which is faster but limits the location of the chromium for the carbides to regions near the grain boundaries. The carbon diffuses by interstitial diffusion, which is much faster and easier than substitutional or again along the boundaries.
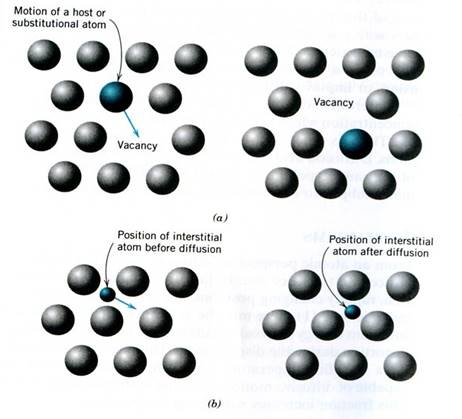
1.Vacancy controlled also Substitutional – a vacancy or missing atom on its lattice site is needed for the atom to jump into. As one atom jumps into a vacancy it leaves a vacancy.
2. Interstitial diffusion involves small atoms diffusing in a matrix of large atoms. For metallic atoms, the H, C, N, O, B atoms are the ones small enough to fit in the spaces
between the large metallic atoms.
3. Grain boundary diffusion uses defect structure of grain boundary so much faster than vacancy or substitutional diffusion rates
Steady State Diffusion – constant linear concentration gradient.
J – Diffusion Flux – atoms/m2.sec – number of atoms moving through one m2 in one sec.
This is the rate of unidirectional mass motion per unit area per second.
J= -D(dC/dx) Ficks First Law
D – diffusivity or diffusion coefficient – m2/s. Depends on temperature, diffusing element,
bond strength, packing factor and imperfections.
dC/dx – concentration gradient.
Solute Solvent D at 500 C D at 1000 C Type
Carbon BCC iron 5x10-12 2x10-9 Interstitial
Carbon FCC iron 5x10-15 3x10-11 Interstitial
Iron BCC iron 10-20 3x10 -14 Self diffusion
Nickel FCC iron 10-23 2x10-16 Substitutional
Silver Silver Xtal 10-17 Self
Silver Silver Grain Bound 10-11 Effect of defects
The net effect is that the region adjacent to the grain boundaries becomes depleted in the corrosion resistant element of chromium. In effect a small region of a non- stainless steel is formed as the chromium is removed by second phase formation.
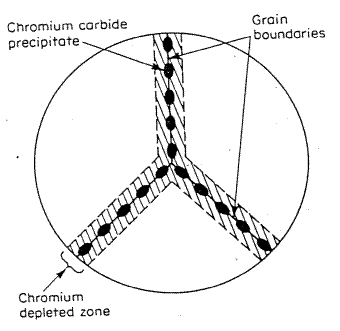

Carbide precipitates on a grain boundary in stainless steels.
In a “sensitized” stainless steel, the whole component is subject to a thermal history which induces carbide formation on grain boundaries uniformly through the stainless steel part.
In weld decay, the thermal history is such that only a local region adjacent to the weld region forms the carbide phase. The fused region where liquid metal exists is too hot and cools to rapidly through the chromium carbide stable region to form. Areas some distance from the weld do not reach the temperature range for carbide formation. It is only the region some distance from the weld that has the correct thermal history for weld decay to occur. For this reason thin sheet less than 1/32" thick is unlikely to suffer weld decay as it cools too quickly. Low power density welding techniques such as torch welding where heat is applied over a wide range will be liable to cause weld decay. High power density techniques such as laser welding will reduce the liability to form carbides as the power and heat input is very concentrated.
Prevention of Weld Decay and Sensitization.
1. Careful welding technique, for example use low carbon welding rods and electric arc welding. Do not use certain forms of brazing, as the commonly use a 1200oF temperature.
2. Quench the material after welding. This is clearly only of use in limited sizes of component.
3. Lower the carbon content of the steel to less than 0.03wt%. These steels are given the addition specification letter of "L" after the AISI number, for example 304L. If the carbon is not present then carbides cannot form.
4. Use stabilized steels. These contain strong carbide formers such as titanium (TiN) or niobium with tantalum(NbC, TaC). For the niobium containing steels , 347 type, Nb+Ta should be x10 of the carbon content of 0.08%C. For the titanium containing steel, 321, the Ti content should be 5 times the carbon content.
Another method of increasing carbon content is during casting of stainless steels. The surface can pick up carbon from casting materials and release. Once corrosion is started it will continue as crevice or pitting corrosion.
Knife Line Attack.
Unfortunately, a new type of corrosion was initiated when stabilized steel were placed into service. KLA occurs in a narrow bend next to the weld and it appears in stabilized steels. The thermal history in this case is also different.
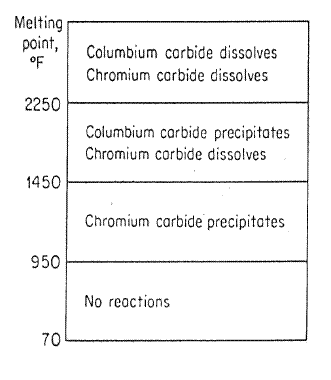
The stability range of carbides is as follows:-
Above 2250oF - All carbides dissolve in solution.
Between 2250 and 1450 - Niobium carbide stable, chromium carbide still in solution.
Between 1450 and 950 - Chromium carbide stable.
Below 950 - no precipitation possible.
In KLA, the metal adjacent to the weld was cooled rapidly from above 2250 to room temperature. No carbides formed in this narrow region, although where the metal was below 2250, niobium carbides were still present. To stress relieve the weld region a stress relief anneal was conducted which was in the region of 950 to 1450. Chromium carbide then formed in the narrow band where the cooling rate after welding was too rapid for the niobium carbide. Corrosion then occurred in this narrow region.
To avoid this hold the complete structure above 1450 to from the desirable carbides. The chromium can then all be used for passive layer formation.
Other alloys can also suffer from specialized forms of intergranular corrosion. One is shown in the figure below, called “Exfoliation Corrosion” and also end grain corrosion. In this case an Al 7057 alloy is in sheet form so been rolled. The resulting microstructure consists of highly elongated grains with precipitate decorated grain boundaries. When these grain boundaries are exposed, by cutting through the sheet, then intergranular corrosion proceeds and results in very thin layers separating in the material.
In the third figure below, a more general intergranular corrosion process in the same alloy is shown, but the grain size is coarser so in this case it is much more like removing pieces of a jigsaw puzzle.
In the Al5XXX series, Al3Mg2 (beta phase) precipitates on grain boundaries and cause sensitization. These form after extended time periods during service.
Exfoliation Corrosion in Al7075

General Intergranular Corrosion in Al7075.
Source: http://che.uri.edu/course/CHE534w/Chap%206.doc
Web site to visit: http://che.uri.edu
Author of the text: indicated on the source document of the above text
If you are the author of the text above and you not agree to share your knowledge for teaching, research, scholarship (for fair use as indicated in the United States copyrigh low) please send us an e-mail and we will remove your text quickly. Fair use is a limitation and exception to the exclusive right granted by copyright law to the author of a creative work. In United States copyright law, fair use is a doctrine that permits limited use of copyrighted material without acquiring permission from the rights holders. Examples of fair use include commentary, search engines, criticism, news reporting, research, teaching, library archiving and scholarship. It provides for the legal, unlicensed citation or incorporation of copyrighted material in another author's work under a four-factor balancing test. (source: http://en.wikipedia.org/wiki/Fair_use)
The information of medicine and health contained in the site are of a general nature and purpose which is purely informative and for this reason may not replace in any case, the council of a doctor or a qualified entity legally to the profession.
The texts are the property of their respective authors and we thank them for giving us the opportunity to share for free to students, teachers and users of the Web their texts will used only for illustrative educational and scientific purposes only.
All the information in our site are given for nonprofit educational purposes