Phase Diagrams are limited in their usefulness because they can only predict the microstructure that will result for equilibrium conditions, i.e. very very slow cooling. Non-equilibrium cooling will result in different microstructures hence altered properties.
Let’s investigate what non-equilibrium cooling do to the microstructure and properties of metals. These non-equilibrium cooling processes are also called heat treatments or thermal processing. They are the temperature vs. time history.
Kinetics is the field of study that takes into account the time dependent aspect of a transformation. TTT diagrams are the tools that we can use to take into account the kinetics of the transformation. They show the relationship between time, temperature and (percent) transformation. There are two types of TTT diagrams:
The IT TTT diagram for eutectoid steel
Recall the eutectoid reaction is: g (0.76wt% C) ® a (0.022wt% C) + q (6.67wt%C)
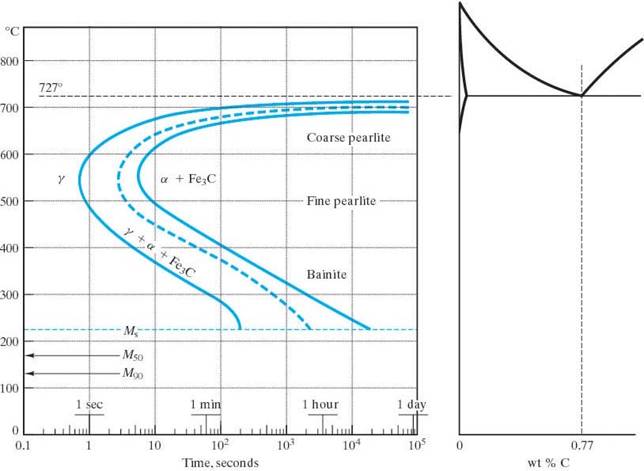
The presence of other alloying elements other than carbon (Cr, Mo, Ni, W, Mn, Si) may cause significant changes in the IT TTT. These include:
Note that each TTT diagram is for a particular composition of steel.
A word about the shape of these “nose” curves (aka shoulder or knee curves):
The nose character is a result of reaction kinetics. The higher temperatures have sufficient diffusion rates for fast transformations, but lack the driving force of being far from the equilibrium temperature at which the reaction takes place. The lower temperatures have a high driving force since there is a great deal of undercooling, however, the diffusion rates are low. Hence it is the intermediate temperatures where the fastest reaction rates occur.
Mictostructures of Steel:
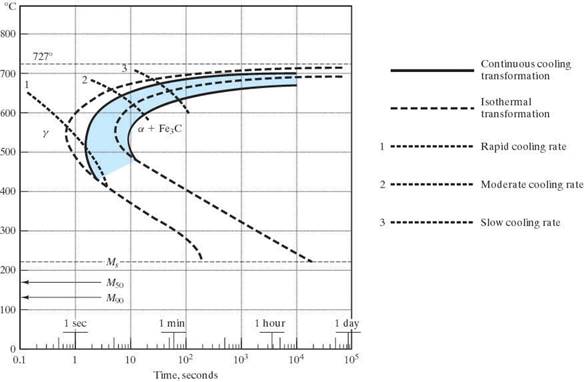
The CCT TTT diagram for eutectoid steel
(Superimposed on an IT for comparison)
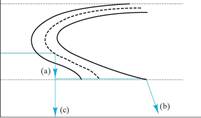
1 2 3 4
Consider the cooling rates 1-4. What will be the resulting microstructures?
Answer:
Note: there will be no bainite with a CCT.
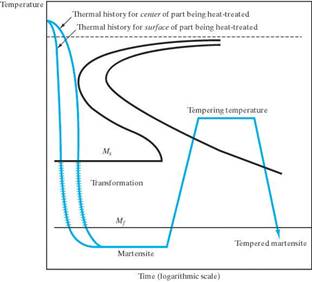
There are some specific heat treatments for steel:
Martempering (marquenching) Austempering What microstructure
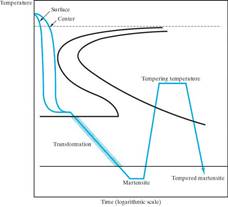
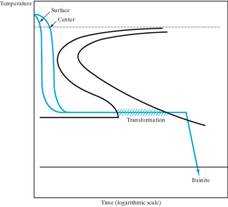
Resulting microstructure Resulting microstructure will result from these
is martensite. is bainite. heat treatments?
In General, we want the steel microstructure to result in Martensite!
This is because martensite is very hard and strong. To get Martensite, of course you must first austenitize and then quench. But to what extent are you assured of a Martensitic microstructure throughout? The factors that determine Martensite formation in a specimen:
The questions to ask are:
Hardenability
 Is the relative ability of steel to be hardened by quenching. It is essentially a measure of the cooling rate at which martensite will form. If martensite will form for slow cooling rates, then the steel has a high hardenability.
Is the relative ability of steel to be hardened by quenching. It is essentially a measure of the cooling rate at which martensite will form. If martensite will form for slow cooling rates, then the steel has a high hardenability.
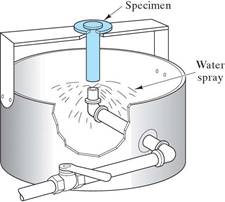
The Jominy End Quench test is used to obtain Hardenability Curves
A Hardenability Curve for a particular steel will give the hardness as a function of cooling rate.
(Cooling rate is correlated to distance from the quenched end, dqe.)
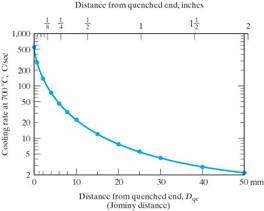
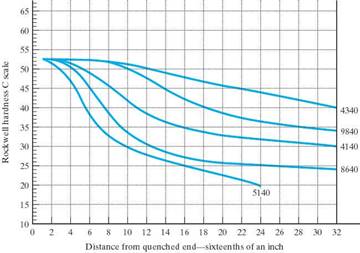
There are plots in the literature that you can use that will give you the cooling rates experienced for any point in a steel specimen of a particular size, geometry and quench. (The standard quenches are water or oil. Once the cooling rate is known, the hardness can be determined from the Hardenability curve.
Note: the larger the surface area to mass ratio of the specimen, the faster the cooling rates will be inside. So irregular shapes may have high hardenabilities.
Precipitation Hardening
aka Age Hardening or Precipitation Strengthening
Precipitation Hardening is a heat treatment in which the strength of an alloy is increased from introducing particles that act as obstacles to slip motion. Not all alloy systems are amenable to this strengthening mechanism. The alloy system must have:
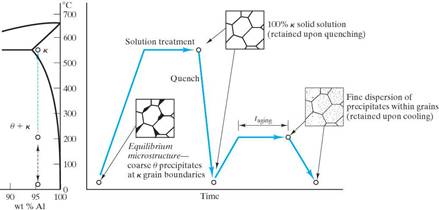
The procedure to produce the microstructure of a precipitation hardened alloy is:
Be careful:
Strain Hardening and Age Hardening can be combined.
Usually the steps are:
Annealing
Annealing is a general term that refers to heating a material and then cooling it. The amount of heating, the time at the elevated temperatures, the rate of cooling are all essential to the final product. Care must be taken during the cooling because of the thermal gradient which might induce plastic deformation, warping, cracking.
For metals an annealing process will increase the ductility and decrease the strength. This could range from merely relieving residual stresses (recovery) to returning it to the condition it had before any plastic deformation was done to it so that it has a new, stress free low energy crystal structure (recrystallization.)
The 3 stages of a full annealing heat treatment:
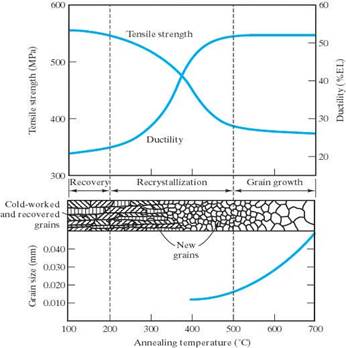 Grain Growth
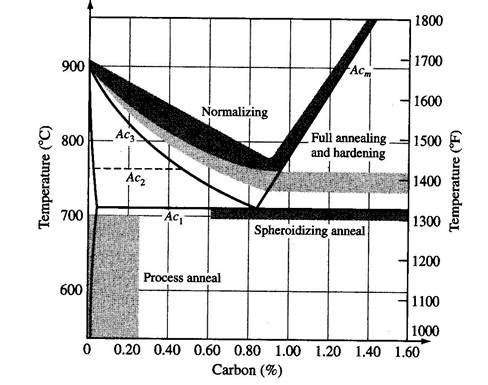
Grain GrowthSome annealing procedures for Ferrous Alloys:
Stress Relief Anneal
Process Anneal
Normalizing
Full Anneal
Spheroidizing
Source: https://fog.ccsf.edu/~wkaufmyn/ENGN45/Course%20Handouts/Chap10_Kinetics-HeatTreatment.doc
Web site to visit: https://fog.ccsf.edu
Author of the text: indicated on the source document of the above text
If you are the author of the text above and you not agree to share your knowledge for teaching, research, scholarship (for fair use as indicated in the United States copyrigh low) please send us an e-mail and we will remove your text quickly. Fair use is a limitation and exception to the exclusive right granted by copyright law to the author of a creative work. In United States copyright law, fair use is a doctrine that permits limited use of copyrighted material without acquiring permission from the rights holders. Examples of fair use include commentary, search engines, criticism, news reporting, research, teaching, library archiving and scholarship. It provides for the legal, unlicensed citation or incorporation of copyrighted material in another author's work under a four-factor balancing test. (source: http://en.wikipedia.org/wiki/Fair_use)
The information of medicine and health contained in the site are of a general nature and purpose which is purely informative and for this reason may not replace in any case, the council of a doctor or a qualified entity legally to the profession.
The texts are the property of their respective authors and we thank them for giving us the opportunity to share for free to students, teachers and users of the Web their texts will used only for illustrative educational and scientific purposes only.
All the information in our site are given for nonprofit educational purposes