What is Thermodynamics?
ABSTRACT: This article describes Thermodynamics; the most important subject in engineering that is based on four laws. To make Thermodynamics more understandable and easy to percept, its different definitions presented below will be demonstrated by artworks. This is a new approach developed by the author that has been applied to subjects in natural sciences, technological sciences, humanities and social sciences as well as life sciences. In future articles, the four laws will be demonstrated by art.
Thermodynamics is an engineering tool used to describe processes that involve changes in different parameters such as temperature, pressure etc., transformation of energy and the relationships between heat and work. It generalizes empirical evidence accumulated in our daily lives into four laws that govern every thing in the universe. The name thermodynamics stems from the Greek words thermos (heat) and dynamos (power), which is most descriptive of the early efforts to convert heat into power. Today the same name is broadly interpreted to include all aspects of energy and energy transformations, including power generation, refrigeration, fluid flow, combustion, and relationships among the properties of matter.
The history of thermodynamics [1] is a fundamental strand in the history of physics, the history of chemistry, and the history of science. Owing to the relevance of thermodynamics in science and technology, its history is finely related with the developments of classical mechanics, quantum mechanics, magnetism and chemical kinetics, as well as fields such as meteorology, information theory, biology, and to technological developments such as the steam engine, internal combustion engine, cryogenics and electricity generation. Essential stages that inherently functioned to stimulate modern thermodynamics, began with the arguments of the 5th century Greek philosopher Parmenides. He postulated that a void in nature, essentially what is now known as a vacuum, could not occur. This statement was disproved conclusively, approximately two thousand years later, when Otto von Guericke built a vacuum pump. Carnot relates additional important stage to the study of efficiency of heat engines. In his 1824 thesis Carnot stated that the efficiency of a heat engine depended only on the temperature difference between it heat source and heat sink and not on the working substance. A decade was passed when Clapeyron developed the relationship between vapor pressure and an unknown function of empirical temperature scale. Clausius later identified this unknown function as the absolute temperature scale. The equation relating the vapor pressure to the absolute temperature is known as the Clausius-Calpeyron equation. It was in 1850 when Clausius published his thesis on the Second Law of thermodynamics that is known as "the Clausius statement." It was this statement that marked the beginning of thermodynamics as a science as Gibbs stated it in the late nineteenth century. A year after publication of this thesis, Thomson formulated explicitly the First and Second Laws of thermodynamics. Thomson had already defined an absolute temperature scale in 1848 and was aware of the 1845 publication by Joule in which Joule had demonstrated the equivalence of heat and work. It was 1865 when Clausius introduced the term "entropy" and stated, "The energy of universe is constant. The entropy of universe tends towards a maximum". Introduction of the term entropy resulted in a new formulation of the Second Law by Clausius. The thesis by Gibbs published in 1875 entitled "On the equilibrium of heterogeneous substances" and his other publications have a special place in thermodynamics of mixtures and phase equilibria. Gibbs extended the science of thermodynamics in a general form to heterogeneous systems with and without chemical reactions. He is also credited for derivation and formulation of completely general equilibrium conditions for various cases. Other early contributors to this branch of thermodynamics are the following: Helmholtz, who in 1882 independent of Gibbs, introduced the concept of free energy and derived the relationship now known as the Gibbs-Helmholtz equation; Duhem, who in 1886, derived the Gibbs-Duhem equation; Planck, who in 1887, divided the changes of state into two classes of thermodynamic processes, namely reversible and irreversible processes; Nernst, who in 1906, published heat theorem, and Carathe'odory, who in
1909, developed a new axiomatic basis of thermodynamics.
A large list of definitions appear in ref. [2] of which the following definitions were selected:
1) The science that describes what is possible and what is impossible during energy conversion processes.
2) The study of the combined effects of heat and motion.
3) The study of the relationships between heat, work, and energy.
4) The science of the conversions between heat and other forms of energy.
As seen, the basic quantities that appear in the definitions are: energy, heat and work. Energy is often defined as the ability to do work [3] and is demonstrated in Fig.1. On the left-hand-side is the artwork of Paul Cezanne (1839-1906) a post-impressionist painter entitled “Still Life with Basket of Apples” [4] demonstrating food. It is a fact of life that food provides the needed energy to the body. On the right-hand-side is a sculpture by an unknown artist demonstrating energy - the ability of the body to do work, i.e. to run.
When a force acts on an object to cause a displacement of it, work is done on the object. This is demonstrated in Fig.2 by the artwork of Vincent van Gogh (1853-1890), a Dutch Post-Impressionist artist. It is entitled “Two Peasants Digging” [5]. And finally heat, symbolized by Q, is the energy transferred from one body or system to another due to a difference in temperature T1 – T2. It is demonstrated in Fig.3 entitled “The Gradation of Fire” [6] painted by Rene Magritte (1898-1967), Belgian surrealist artist.
In the following we demonstrate by different artworks the subject of thermodynamics according to the aforementioned definitions. Fig.4 [7] by the American photographer Walter Wick (b.1953) demonstrates the 1st definition of thermodynamic. At room temperature it is possible that condensation of the vapor occurs spontaneously. However it is impossible that evaporation of the drops will occur spontaneously. Energy must be provided for the evaporation.
Fig.5 [8] is another demonstration of the 1st definition of thermodynamics painted by Jim Warren, an American painter born in 1949 who specialized in fantasy art. The flow downwards is possible spontaneously due to the potential energy of the liquid; however the flow upwards is impossible without providing energy.
Figs.6 and 7 demonstrate the second definition of thermodynamics: the study of the combined effects of heat and motion. On the left-hand-side is an artwork by Rene Magritte (1898-1967), Belgian surrealist painter, entitled “The Discovery of Fire” [9] which represents heat. On the right-hand-side is a bronze sculpture entitled “Van Gogh On The Road” [10] made by the British cartoonist John Coll (1936-2007) that represents motion.
In Fig.7 heat is demonstrated by the sun [11] where motion is presented by the artwork “Sky and Water I” [12] of M.C.Escher (1898-1972), one of the world's most famous graphic artists.
The third definition of thermodynamics, “the study of the relationships between heat and work” is demonstrated in Figs.8 to 11. However, it should be emphasized that how exactly the transformation of heat to work occurs practically is the “business” of technology. The left-hand-side of Fig.8 entitled “Sunrise” [13] that demonstrates heat was painted by Roy Lichtenstein (1923-1997), an American Pop artist. The right-hand-hand artwork by an unknown artist represents work.
Fig.9, left-hand-side, entitled “The Fire” [14] that creates heat was painted by Giuseppe Arcimboldo (1527-1593) an Italian painter best known for creating imaginative portrait heads made entirely of such objects as fruits, vegetables, flowers, fish, and books. The work function on the right-hand-side is demonstrated by the painting entitled “Lifting Weights with One Arm” [15] of Eugene Jansson (1862-1915), a Swedish painter. Additional demonstration of heat is shown in Fig.10 on the left-hand-side. It is entitled “Eruption”[16] and was painted by the polish surrealist artist Jacek Yerka born in 1952. Work is demonstrated on right-hand-side [17] by the famous image of Sisyphus who was punished to push stone upwards infinite times. From here comes the expression sissify work meaning doing work which yields nothing. And finally in Fig.11, the combination between heat and work in a single artwork, is demonstrated by the artwork of Vincent van Gogh (1853-1890) a Dutch expressionist. It is entitled “Sower” [18].
CONCLUSIONS
It is well known that thermodynamics is not an easy subject in chemical engineering studies. However, from his experience of teaching thermodynamics, the author believes that presentation by artworks of what is thermodynamics makes this subject clearer, more understandable and easier to percept. The author recommends that this article may be used to present “what is thermodynamics” in the first lecture of thermodynamics.
REFERENCES
[1] http://en.wikipedia.org/wiki/History_of_thermodynamics
[2]http://www.google.co.il/search?hl=iw&defl=en&q=define:Thermodynamics&sa=X&oi=glossary_definition&ct=title
[3] http://en.wikipedia.org/wiki/Energy
[4] http://blog.360.yahoo.com/blog-cM5BH9g.dqc8reyqv.K710U-?cq=1&p=298
[5] http://wahooart.com/A55A04/w.nsf/Opra/BRUE-5ZKGTY?OpenDocument&ChangeLangue=ES
[6] “Rene Magritte” by A.M Hammacher, Thames and Hudson, 1986
[7] “A Drop of Water” by Walter Wick, Scholastic Press/New York, 1997
[8] http://images.google.co.il/images?gbv=2&ndsp=18&hl=iw&q=Jim+Warren&start=36&sa=N
[9] “Rene Magritte 1898-1967” by Marcel Paquet, Benedict Taschen Verlag GmbH, 1994
[10] http://www.thekennygallery.ie/exhibitions/1998/colljohn/
[11] http://www.swpc.noaa.gov/primer/primer_graphics/Sun.png
[12] “The World of M.C.Escher” edited by J.L.Locher, Harry N.Abrams, Inc., Publishers, New York, 1971
[13] http://images.easyart.com/i/prints/rw/en_easyart/lg/3/3/Sunrise-Roy-Lichtenstein-33065.jpg
[14] http://images.google.co.il/imgres?imgurl=http://www.spamula.net/blog/i16/arcimboldo10.jpg&imgrefurl=http://www.spamula.net/blog/2004/03/arcimboldos_elements.html&h=1848&w=1390&sz=512&hl=iw&start=6&tbnid=H-zJFDd0YEqAxM:&tbnh=150&tbnw=113&prev=/images%3Fq%3Darcimboldo%26gbv%3D2%26hl%3Diw%26sa%3DG
[15] http://images.google.co.il/imgres?imgurl=http://upload.wikimedia.org/wikipedia/commons/a/ac/Jans
[16] “Mind Fields” by Jacek Yerka, Morpheus International, 1994
[17] http://images.google.co.il/imgres?imgurl=http://www.cs.uni.edu/~wallingf/blog-images/art/sisyphus
[18] http://ayeshahauer.com/photos/VanGoghSower.jpg
Source: http://web.nchu.edu.tw/pweb/users/cmchang/lesson/5497.doc
Web site to visit: http://web.nchu.edu.tw
Author of the text: indicated on the source document of the above text
Unit 1: FUNDAMENTAL CONCEPTS AND DEFINITIONS
INTRODUCTION:
Thermodynamics is the science of energy transfer and its effect on the physical properties of substances. The alternate definition is: thermodynamics is the science that deals with work and heat and these properties of substances that bear a relation to heat and work. Like all sciences, the basis of thermodynamics is experimental observation.
Thermodynamics (from the Greek therme, meaning "heat" and dynamis, meaning "power") is a branch of physics that studies the effects of changes in temperature, pressure, and volume on physical systems at the macroscopic scale by analyzing the collective motion of their particles using statistics
This subject was developed mainly by
1) Carnot 2) Mayer 3) Clausius 4) Joule
5) Kelvin 6) Maxwell 7) Plank 8) Gibbs
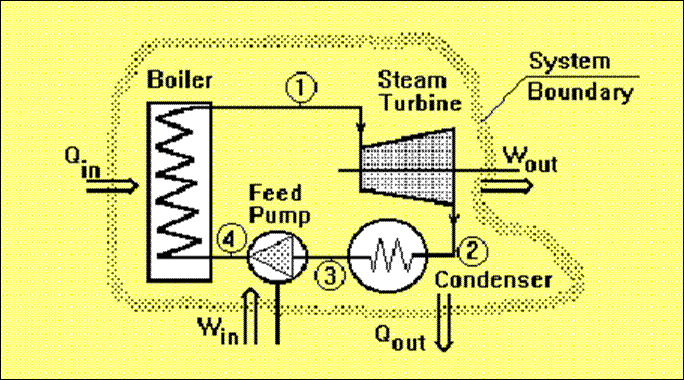
A thermodynamic system is a device or combination of devices containing a quantity of matter that is being studied. A typical thermodynamic system - heat moves from hot (boiler) to cold (condenser), and work is extracted, in this case by a series of pistons.
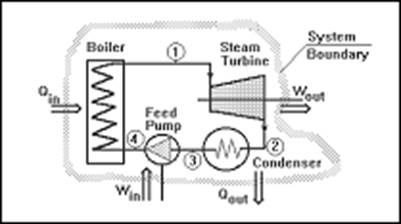
A typical thermodynamic system
The study of thermodynamics is the basis of such fields as steam power plants, IC Engines, Gas dynamics and aerodynamics, fluid mechanics, Refrigeration and Air conditioning and heat transfer.
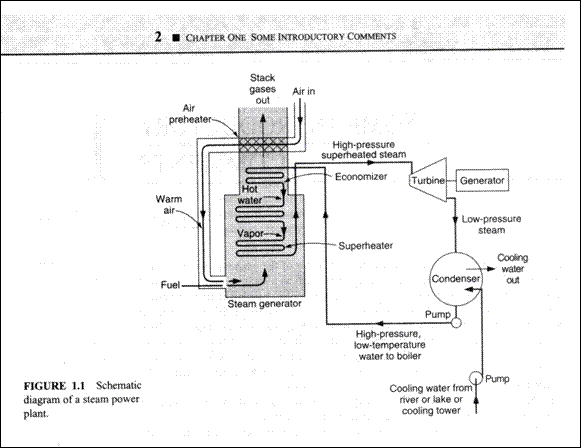
A Steam Power Plant
Thermodynamics deals with four laws. Namely Zeroth law, first law, second law
and Third law of thermodynamics. Fortunately, there is no mathematical proof for any of these laws of thermodynamics, like physical laws, but they are deduced from experimental observations.
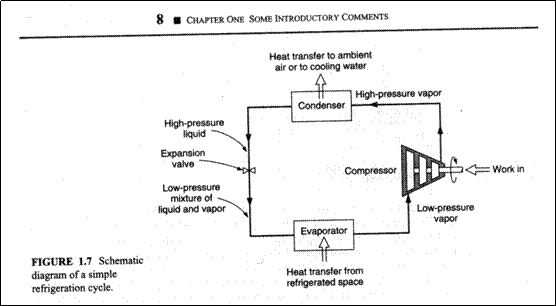
Refrigeration Cycle
Thermodynamics deals with three E’s, namely Energy, Equilibrium and Entropy. Thermodynamics also talks about study of materials, chemical reactions, plasmas and other biological reactions.
Macroscopic and Microscopic point of view:
This study deals with macroscopic, as opposed to microscopic or statistical thermodynamics. In microscopic thermodynamics individual molecule is considered and analysis of collective molecular action. In macroscopic thermodynamics, we concern ourselves with the overall effect of the individual molecular interaction.
Macroscopic point of view: The macroscopic level is the level on which we live. We measure most of the quantities on this level.
Ex: Temperature measurement, pressure measurement, total volume measurement, specific volume measurement. Thus, microscopic point of view will be used only to explain some phenomena that can’t be understood by macroscopic means.
Microscopic point of view: Considers a system containing a cube of 25 mm containing monoatomic gas at atmospheric pressure and temperature. This volume contains approx. 1020 atoms. To describe the position of each atom in a coordinate system we require three equations. To describe the velocity of each atom we have to specify 3 velocity components. Thus to describe completely the behavior of the system from a microscopic point of view we must deal at least 6*1020 equations. It is a hopeless computational task.
The other approach that reduces number of variables to a few that can be handled is the macroscopic point of view of Classical Thermodynamics. It concerns with the gross or average effect of many molecules and can be measured by instruments. This measurement is the time-averaged influence of many molecules.
In the present study, we concentrate on macroscopic point of view.
Statistical thermodynamics, classical thermodynamics deals with the significance of microscopic point of approach. From the macroscopic point of view it is very clear that continuum has to be there in the system because we are not concerned with the behavior of the individual molecule.
Substance: What follows will be illustrations of the thermodynamics, one must be able to solve problems and to do what the part of the problem must be enumerated. The first consideration is that there must be something performing the energy transformations. This something is called a substance. Ex: In case of IC engine gasoline and air mixture constitutes the substance. In steam turbine the substance is steam.
The substance may be further divided into sub categories, namely pure substance i.e. if it is homogeneous in nature- i.e. if it does not undergo chemical reaction and is not a mechanical mixture of different spices. The other substance is a mixture substance which is not a pure substance.
Thermodynamic System:
A substance does not exist alone. It must be contained. This brings us to the concept of a system.
In thermodynamics a system is defined as any collection of matter or space of fixed identity, the concept is one of the most important thermodynamics.
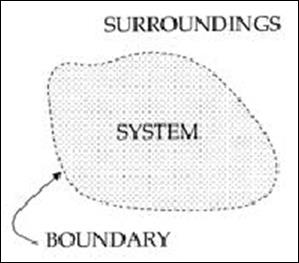
Concept of a Boundary
System boundary: When a system is defined, let us say, fluid in a cylinder, what separates the fluid from the cylinder wall and the piston and everything external to the piston-cylinder? it is the system boundary. Everything not in the system is called the surrounding. Note that piston can be raised or lowered, but the system, matter of fixed identity is constant.
The system is further divided into closed system, open system and isolated system.
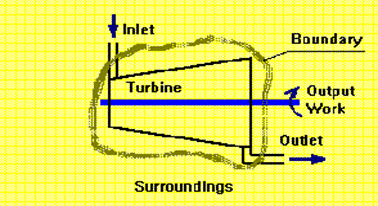
A Thermodynamic system is defined as a quantity of matter or a region in space upon which attention is concentrated in the analysis of a problem. Everything external to the system is called the surrounding or environment. The system is separated from the surrounding by the system boundary. Boundary may be either fixed or moving. A system and its surrounding together comprise a universe.
A System, Surroundings and Boundary
Open System: The open system is one in which matter crosses the boundary of the system. There may be energy transfer also. Most of th4e engineering devices are generally open systems. Ex: An air compressor in which air enters at low pressure and leave at high pressure and there is energy transfer across the system boundary.
An Open System
Closed System: A closed system is a system of fixed mass. There is no mass transfer across the system boundary. Ex: A certain quantity of fluid in a cylinder bounded by a piston constitutes a closed system.
A Closed System
Isolated System: The isolated system is one in which there is no interaction between the system and surrounding. It is of the fixed mass and energy and there is no mass or energy transfer across the system boundary.
Control Volume and Control Surface:
In thermodynamic analysis of an open system such as air compressor, gas turbine in which there is a flow of mass into and out of the system, attention is focused on a certain volume in space surrounding the compressor known as control volume, bounded by a surface called the control surface. Matter as well as energy can cross the control surface.
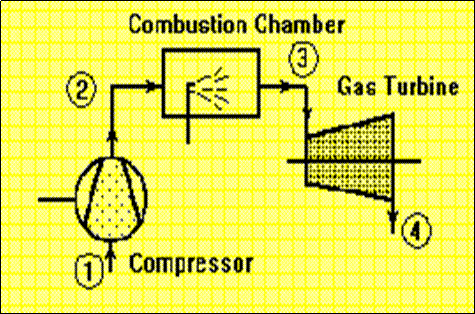
Gas turbine
Homogeneous and Heterogeneous system:
A quantity of matter homogeneous throughout in chemical composition and physical structure is called a phase. Every substance can exist in any one of the three phases viz. Solid, Liquid or gas.
A system consisting of a single phase is called a homogeneous system while a system consisting of more than one phase is known as a heterogeneous system.
Thermodynamic properties are taken from a macroscopic perspective. We are dealing with quantities that can either directly or indirectly be measured or counted. Therefore, the matter of units becomes an important consideration.
Mass, length and time are considered as fundamental physical quantities, they are related by Newton's second law of motion, which states that the force acting on a body is proportional to the product of mass and acceleration in the direction of force.
i.e. F= m * a
Mass – kg Length – m Time – s
This is adopted by CGPM- General Conference of Weights and Measures
In thermodynamics temperature is also considered as fundamental unit in Kelvin.
Energy: One of the very important concepts in a study of thermodynamics is the concept of energy. It is defined as the capacity to do work. It is also defined as the capability to produce an effect
When considered from molecular point of view, three general forms of energy become important.
1) Intermolecular potential energy.
2) Molecular kinetic energy
3) Intermolecular energy.
The energy is the important concept which depends on the mass, velocity, intermolecular attraction. In all intermolecular internal energy is most difficult to evaluate.
Specific Volume: It is a macroscopic property and defined as the volume occupied by unit mass. It is reciprocal of density and its unit is m3/ kg. The specific volume of a system in a gravitational field may vary from point to point. Specific volume increases as the elevation increases. Thus the definition of specific volume involves the specific volume of a substance at a point in a system.
Pressure: The pressure in a fluid at rest at a given point is the same in all directions. We define pressure as the normal component of force per unit area. Its unit is pascal or N/m2. When dealing with liquids and gases we ordinarily speak of pressure. For solids we speak of stresses.
Two other units not part of international system continue to be widely used are
Bar = 105 Pa = 0.1MPa and standard atmosphere is 1 atm = 101325 Pa.
In most thermodynamic investigations, we are concerned with absolute pressure. Most pressure vacuum gauges however read the difference between the absolute pressure and the atmospheric pressure at the gauge. This refers to as gauge pressure.
Temperature: It is a fundamental property of thermodynamics and defined as the hotness of the body. Temperature first of all as a sense of hotness or coldness when we touch an object. We also learn that when a hot body and a cold body are brought into contact the hot body becomes cooler and cold body becomes warmer. Because of these difficulties in defining temperature we define equality of temperature.
Property: It is defined as any quantity that depends on the state of the system and is independent of the path ( i.e. the prior history) by which the system arrived at the given state. Conversely the state is specified or described by the properties and later we will consider the number of independent properties a substance can have, i.e, the minimum number of properties that must be specified to fix the state of a substance.
Thermodynamic properties can be divided into 2 general classes: intensive and extensive properties:
Intensive property: An intensive property is independent of mass; thus intensive property value remains same even if the matter is divided into two equal parts. Ex: pressure, temperature, density etc.
Extensive Property: The value of an extensive property varies directly with the mass, i.e. if a quantity of matter in given state is divided into 2 equal parts, the properties will have the half the original values. Ex: mass, total volume, total enthalpy, total energy etc.
The state may be identified or described by certain observable macroscopic properties; some familiar one are temperature, pressure and density. The state is specified or described by the properties. The state point can be indicated on a thermodynamic coordinate system. Thermodynamic coordinate system includes pressure volume diagram, temperature volume diagram, temperature entropy diagram, enthalpy entropy diagram, pressure enthalpy diagram so on and so forth.
These co-ordinate systems represent thermodynamic state of a substance. Consider a system not undergoing any change. At this point all the properties can be measured or calculated throughout the entire system, which gives us a set of properties that completely, describes the condition or the state of the system.
Thermodynamics deals with equilibrium state. When a system undergoes any change then change of state will occur.
Piston Cylinder arrangement
Process: Whenever one or more of the properties of a system change we say that a change in state has occurred. For ex: in a piston and cylinder arrangement, if weight is removed from the piston rises and change in state occurs in which pressure decreases and specific volume increases. The path of succession of states through which the system passes is called process.
Path: Path is the complete series of states through which the system passes during a change from one given state to other state. It is clear that the transformation of a system from one fixed state to another state is called a process.
The thermodynamic processes that are commonly met within engineering practice are 1) Constant pressure process (Isobaric) 2) Constant volume process(Isochoric) 3)Constant temperature process( Isothermal) 4) Reversible adiabatic process (Isentropic process) 5) Polytropic, process 6) Throttling process.
If the system passes through a series of equilibrium states during the process it’s called reversible process. On the other hand the system passes through a series of non-equilibrium states during a process it is called irreversible process. The state of the processes cannot be plotted on the co-ordinate systems since the path of the process is not defined.
Generally the system is in equilibrium in the beginning and at the end of the process, the reversible process can be plotted on the coordinate diagram by continuous line and an irreversible process by a dotted line.
Representation of a reversible process An irreversible process
Quasi – static process (very slow process) :
Quasi – meaning almost, static meaning infinite slowness. Thus quasi-static process is infinitely slow transition of a system. Infinite slowness is the characteristic feature of a quasi-static process. A quasi-static process is a succession of equilibrium states. It is a reversible process.
Cycle: It is a process whose initial and final states are same. Thus at the end of a cycle all the properties of a working fluid have the same values as they had in the initial states.
There are 2 types of cycles. viz. thermodynamic cycle and mechanical cycle.
Thermodynamic cycle:
It is one in which the working substance is re circulated. Ex: water that circulates through steam power plant and refrigerant that passes through refrigeration plant are the examples of thermodynamic cycle. There is change of phase during the process but the end states do not change
Mechanical cycle:
In case of a mechanical cycle the working substance is not re circulated. In an IC engine air and fuel are burnt in the engine, converted into the products of combustion and are then exhausted into the atmosphere. Hence this type of cycle is called mechanical cycle.
Thermodynamic Equilibrium:
The word equilibrium implies a state of balance. In an equilibrium state, there are no unbalanced potentials within the system or driving forces. Thus, a system in equilibrium experiences no changes when it is isolated from its surroundings.
There are many types of equilibrium. A system is not in thermodynamic equilibrium unless the condition of all the relevant types of equilibrium are satisfied, which includes 1) Thermal equilibrium 2) Mechanical equilibrium 3) Phase equilibrium and 4) Chemical equilibrium.
Thermal Equilibrium:
If the temperature is the same throughout the entire system .i.e the system involves no temperature differential which is the driving force or heat flow then we say system is in thermal equilibrium.
Mechanical equilibrium:
It is related to pressure, velocity. A system is in mechanical equilibrium if there is no change in pressure, velocity, specific volume at any point of the system with respect to time. However the pressure may vary within the system with elevation as well as resultant of gravitational effects. However there should not be any imbalance of forces. Then we say the system is in mechanical equilibrium
Phase equilibrium:
If a system involves two phases it is in phase equilibrium when the mass of each phase reaches equilibrium level and stays there.
Chemical equilibrium:
If the systems chemical composition does not change with time, i.e., no chemical reaction occur then we say the system is in chemical equilibrium.
Thus if all thermal, mechanical, phase and chemical equilibrium exist for a system then we say the system exist in thermodynamic equilibrium
Diathermic wall:
A wall which is impermeable to the flow of heat is an adiabatic wall, where as a wall which permits the flow of heat is a diathermic wall. Thus heat flow takes place through this wall.
Zeroth law of Thermodynamics:
It states that when two bodies have equality of temperature with the third body, they in turn have equality of temperature with each other.
When a body A is in thermal equilibrium with body B and also separately with body C then B and C will be in thermal equilibrium with each other.
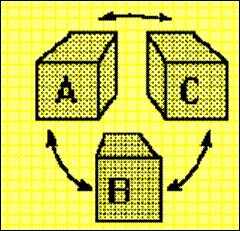
Equality of temperature – Zeroth law of Thermodynamics.
Temperature scales:
Two scales are commonly used for measurement of temperature namely, Fahrenheit after Gabriel Fahrenheit (1686-1736) and Celsius. The Celsius scale was formerly called the centigrade scale but is now designated the Celsius scale after Anders Celsius (1701-1744), the Swedish astronomer who devised this scale.
In SI units of temperature scale we use absolute temperature scale or absolute scale of temperature which comes from second law of thermodynamics and its unit is Kelvin.
Table: Thermometers and Thermometric Properties
The absolute scale is related the Celsius scale is the Kelvin scale after William Thompson, 1824-1907, who is also known as Lord Kelvin and is designated K. The relation is K=oC + 273.15
In 1967 the CGPM defined the Kelvin as 1/273.16 of the temperature at the triple point of water.
Thermometer |
Thermometric property |
Symbol |
Constant volume gas thermometer |
Pressure |
P |
Constant pressure gas thermometer |
Volume |
V |
Electrical Resistance thermometer |
Resistance |
R |
Thermocouple |
Thermal e.m.f. |
e |
Mercury in glass thermometer |
Length |
L |
In order to obtain a quantitative measure of temperature a reference body is used, and a certain physical characteristic of this body which changes with temperature is selected. The changes in the selected characteristic may be taken as an indication of change in temperature. The selected characteristic is called the thermometric property and the reference body which is used in the determination of temperature is called the thermometer.
A Very common thermometer consists of a small amount of Mercury in an evacuated capillary tube. In this case the extension of the mercury in the tube is used as the thermometric property.
Presently temperature of triple point of water which is an easily reproducible state is now the standard fixed point of thermometry.
International practical temperature scale: an international temperature scale was adopted at the seventh general conference on weights and measures held in 1927. It was not to replace the Celsius or ideal gas scales, but to provide a scale that could be easily and rapidly used to calibrate scientific and industrial instruments.
International practical scale agrees with Celsius scale at the defining fixed points listed in following table.
|
Temperature in 0C |
Normal boiling point of oxygen |
-182.97 |
Triple point of water (standard) |
+0.01 |
Normal boiling point of water |
100.0 |
Normal boiling point of sulphur |
444.60 |
Normal melting point antimony |
630.50 |
Normal melting point of silver |
960.80 |
Normal melting point of gold |
1063.0 |
Numerical Examples with Solutions:
1) A tank contains mixture of 20kg of nitrogen and 20 kg of carbon monoxide. The total tank volume is 20m3. Determine the density and specific volume of the mixture.
Solution:
Total mass of the mixture: 20 kg N2 + 20 kg CO = 40 kg mixture
Specific volume = volume / mass = 20 m3 / 40kg = 0.5 m3/kg
2) An automobile has a 1200 kg mass and is accelerated to 7m/s2. Determine the force required to perform this acceleration.
Solution:
Force required F = m * a = 1200 * 7 =8400 kg m /s2 = 8400 N. Ans.
3) The emf in a thermocouple with the test junction at t0C on gas thermometer scale and reference junction at ice point is given by e = 0.20 t – 5 * 10-4 t2 m V. the milli voltmeter is calibrated at ice and steam points. What will this thermometer read in a place where gas thermometer reads 500C?
Solution:
At ice point, when t = 0oC, e= 0 mV.
At steam point, when t=100oC, e= 0.20 * 100 – 5* 10-4 * (100)2 = 15 mV
At t = 50oC, e = 0.20 * 50 – 5* 10-4 * (50)2 = 15 mV = 8.75 mV
When the gas thermometer reads 50oC, the thermocouple will read
t = 100/15 * 8.75 or 58.33 oC. Ans.
4) A barometer to measure absolute pressure shows a mercury column height of 725mm. The temperature is such that the density of the mercury is 13550 kg/m3. Find the ambient pressure.
Solution:
Ambient pressure = ρ * g * h = 13550 * 9.81* 725 / 1000 = 96371Pa
or P = 0.9637 bar. Ans.
5) The temperature t on a Celsius thermometric scale is defined in terms of a property p by the relation p = e(t-B)/A, where A and B are constants. Experiment gives values of p of 1.86 and 6.81 at the ice and steam point respectively. Obtain relation for t and also find the temperature t for the reading of p = 2.5
Solution:
At ice point, t = 0oC, p= 1.86 Hence 1.86 = e(0-B)/A
1.86 = e –B/A or eB/A = 1/1.86 and ln eB/A = ln 1/1.86
B/A = - 0.62058 --------------- (1)
At steam point, t=100oC, p= 6.81 6.81 = e(100-B)/A
ln 6.81 = (100 - B) /A = 1.9184
1.9184 A + B =100--------------------------(2)
Solving equations (1) and (2)
A = 77.05 and B = -47.81 hence p = e [t – (-47.81) ] / 77.05
The temperature at p=2.5, 2.5 = e (t+47.81)/77.05
ln 2.5 =( t + 47.81) / 77.05 or t + 47.81 = 0.9163 77.05
or t = 22.79 0C Ans.
6) A hiker is carrying a barometer that measures 101.3 kPa at the base of the mountain. The barometer reads 85 kPa at the top of the mountain. The average air density is 1.21kg/m3. Determine the height of the mountain.
Solution:
Pressure at the base of the mountain = ρ1 * g * h1.
h1= p / (ρ1 * g ) = 101.3 *1000 / (9.81 * 1.21) = 8534 m.
Also at the top of the mountain p = ρ2 * g * h2.
h2 = p / (ρ2 * g ) = 85 *1000 / (9.81 * 1.21) = 7161 m.
Hence the height of the mountain = h1- h2 = 8534 – 7161= 1373 m. Ans.
7) A lunar excursion module (LEM) weighs 1500 kgf at sea level; on earth. What will be its weight on the surface of the moon, where g =1.7 m/s2? On the surface of the moon, what will be the force in kgf required to accelerate the module at 10m/s2?
Solution:
The mass m of the LEM is given by W = mg/g0
1500 kgf = m * { (9.806 m/s2 ) / ( 9.806 kg/ kgf * m/s2 )}
i.e. m=1500 kg
The weight of the LEM on the moon would be
W = 1500 kg * {(1.7 m / s2) / (9.806 kg / kgf * m/s2 ) } Ans.
The force required to accelerate the module at 10 m/s2
= [ 1500 kg / (9.806 kg/kgf * m/s2) ] * 10 m/s2 = 1530 kgf Ans.
8) A cannon ball of 5 kg acts on a piston in a cylinder of 0.15m diameter. As the gunpowder is burnt, a pressure of 7 MPa is created in the gas behind the ball. What is the acceleration of the ball if the cylinder is pointing horizontally?
Solution:
Mass of the cannon ball = 5 kg
Diameter of the cylinder = 0.15 m
Force = Area * Pressure = (π / 4) * (0.15)2 *7* 106 = 123716.25 N Ans.
F = m*a Hence a = F /m = 123716.25 / 5 = 24743.25 m / s2 Ans.
Source: http://jnnce.ac.in/jnndemo/coursematerials/Unit%201.docx
Web site to visit: http://jnnce.ac.in/
Author of the text: indicated on the source document of the above text
If you are the author of the text above and you not agree to share your knowledge for teaching, research, scholarship (for fair use as indicated in the United States copyrigh low) please send us an e-mail and we will remove your text quickly. Fair use is a limitation and exception to the exclusive right granted by copyright law to the author of a creative work. In United States copyright law, fair use is a doctrine that permits limited use of copyrighted material without acquiring permission from the rights holders. Examples of fair use include commentary, search engines, criticism, news reporting, research, teaching, library archiving and scholarship. It provides for the legal, unlicensed citation or incorporation of copyrighted material in another author's work under a four-factor balancing test. (source: http://en.wikipedia.org/wiki/Fair_use)
The information of medicine and health contained in the site are of a general nature and purpose which is purely informative and for this reason may not replace in any case, the council of a doctor or a qualified entity legally to the profession.
The texts are the property of their respective authors and we thank them for giving us the opportunity to share for free to students, teachers and users of the Web their texts will used only for illustrative educational and scientific purposes only.
All the information in our site are given for nonprofit educational purposes