Solid materials have been conveniently grouped into three basic classifications: metals, ceramics, and polymers. This scheme is based primarily on chemical makeup and atomic structure, and most materials fall into one distinct grouping or another, although there are some intermediates. In addition, there are the composites, combinations of two or more of the above three basic material classes. A brief explanation of these material types and representative characteristics is offered next. Another classification is advanced materials—those used in high- technology applications—viz. semiconductors, biomaterials, smart materials, and nanoengineered materials.
Materials in this group are composed of one or more metallic elements (such as iron, aluminum, copper, titanium, gold, and nickel), and often also nonmetallic elements (for example, carbon, nitrogen, and oxygen) in relatively small amounts. Atoms in metals and their alloys are arranged in a very orderly manner and in comparison to the ceramics and polymers, are relatively dense (Figure 1.1).With
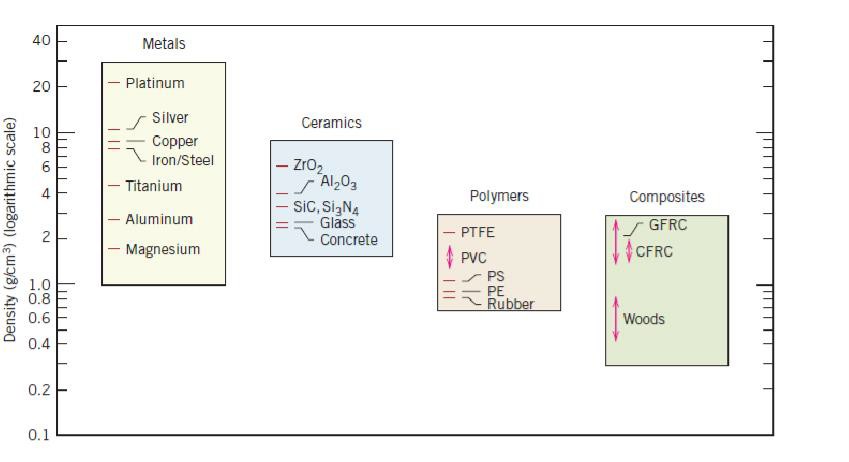
Bar-chart of room temperature density values for various metals, ceramics, polymers, and composite materials
regard to mechanical characteristics, these materials are relatively stiff (Figure 1.2) and strong (Figure 1.3), yet are ductile (i.e., capable of large amounts of deformation without fracture), and are resistant to fracture (Figure 1.4), which accounts for their widespread use in structural applications. Metallic materials have large numbers of nonlocalized electrons; that is, these electrons are not bound to particular atoms. Many properties of metals are directly attributable to these electrons. For example, metals are extremely good conductors of electricity (Figure 1.5) and heat, and are not transparent to visible light; a polished metal surface has a lustrous appearance. In addition, some of the metals (viz., Fe, Co, and Ni) have desirable magnetic properties.
Figure 1.6 is a photograph that shows several common and familiar objects that are made of metallic materials.
Ceramics are compounds between metallic and nonmetallic elements; they are most frequently oxides, nitrides, and carbides. For example, some of the common ceramic
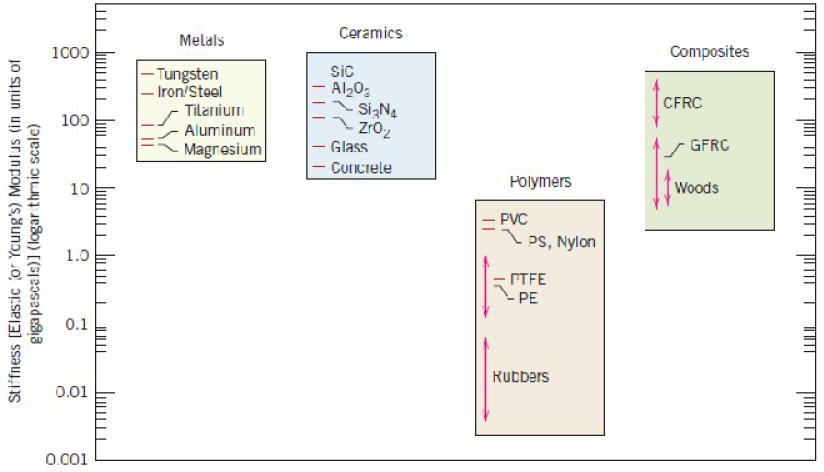
Bar-chart of room temperature Stiffness (i.e., elastic modulus) values for various metals, ceramics, polymers, and composite materials.
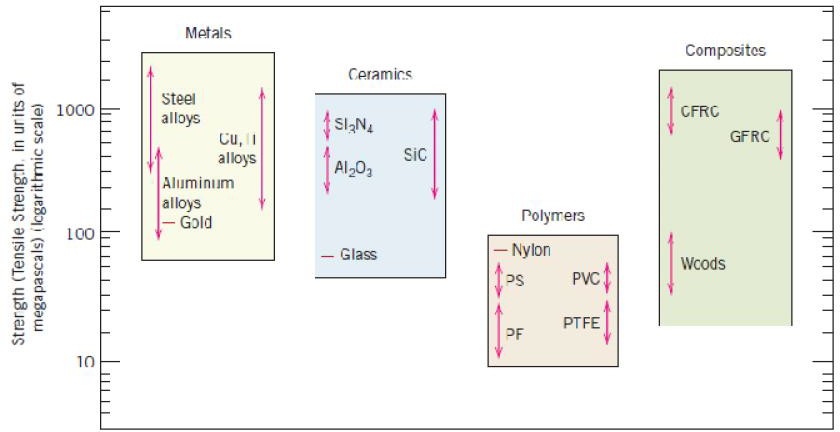
Bar-chart of room temperature strength (i.e., tensile strength) values for various metals, ceramics, polymers, and composite materials
materials include aluminum oxide (or alumina,Al2O3), silicon dioxide (or silica, SiO2), silicon carbide (SiC), silicon nitride (Si3N4), and, in addition, what some refer to as the traditional ceramics—those composed of clay minerals (i.e., porcelain), as well as cement, and glass. With regard to mechanical behavior, ceramic materials are relatively stiff and strong—stiffnesses and strengths are comparable to those of the metals (Figures 1.2 and 1.3). In addition, ceramics are typically very hard. On the other hand, they are extremely brittle (lack ductility), and are highly susceptible to fracture (Figure 1.4). These materials are typically insulative to the passage of heat and electricity (i.e., have low electrical conductivities, Figure 1.5), and are more resistant to high temperatures and harsh environments than metals and polymers. With regard to optical characteristics, ceramics may be transparent, translucent , or opaque
(as shown in figure (A)), and some of the oxide ceramics (e.g., Fe3O4) exhibit magnetic behavior.
Photograph of three thin disk specimens of aluminum oxide, which have been placed over a printed page in order to demonstrate their differences in light-transmittance characteristics.
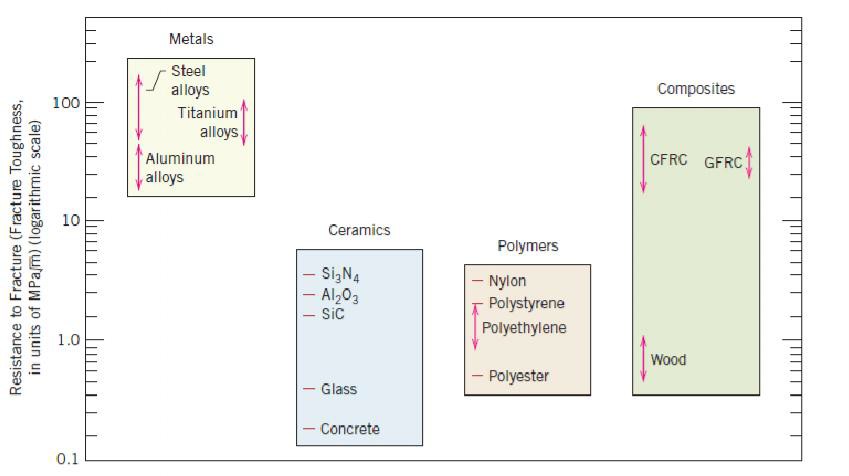
Bar-chart of room-temperature resistance to fracture (i.e., fracture toughness) for various metals, ceramics, polymers, and composite materials.
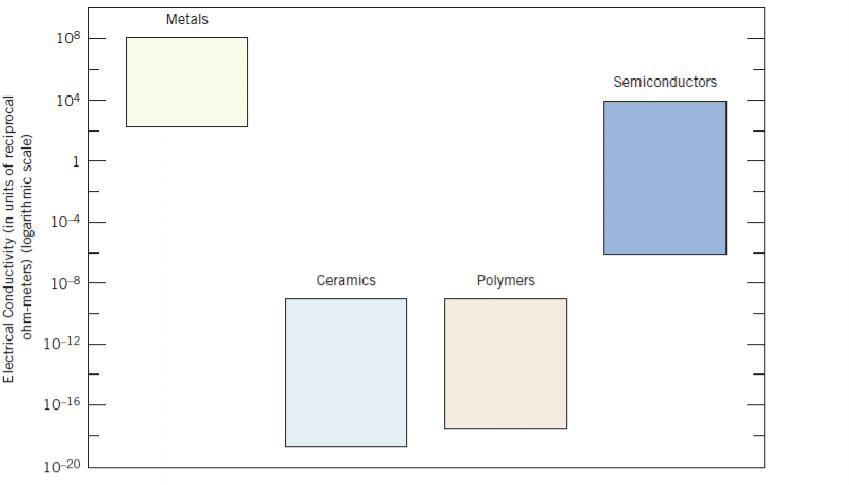
Bar-chart of room temperature electrical conductivity ranges for metals, ceramics, polymers, and semiconducting materials.
Polymers include the familiar plastic and rubber materials. Many of them are organic compounds that are chemically based on carbon, hydrogen, and other nonmetallic elements (viz.O,N, and Si). Furthermore, they have very large molecular structures, often chain-like in nature that have a backbone of carbon atoms. Some of the common and familiar polymers are polyethylene (PE), nylon, poly(vinyl chloride) (PVC), polycarbonate (PC), polystyrene (PS), and silicone rubber. These materials typically have low densities (Figure 1.1), whereas their mechanical characteristics are generally dissimilar to the metallic and ceramic materials—they are not as stiff nor as strong as these other material types (Figures 1.2 and 1.3). However, on the basis of their low densities, many times their stiffnesses and strengths on a per mass.
Familiar objects that are made of metals and metal alloys: (from left to right) silverware (fork and knife), scissors, coins, a gear, a wedding ring, and a nut and bolt.
Common objects that are made of ceramic materials: scissors, a china tea cup, a building brick, a floor tile, and a glass vase.
basis are comparable to the metals and ceramics. In addition, many of the polymers are extremely ductile and pliable (i.e., plastic), which means they are easily formed into complex shapes. In general, they are relatively inert chemically and unreactive in a large number of environments. One major drawback to the polymers is their tendency to soften and/or decompose at modest temperatures, which, in some instances, limits their
use. Furthermore, they have low electrical conductivities (Figure 1.5) and are nonmagnetic.
The photograph in Figure 1.8 shows several articles made of polymers that are familiar to the reader.
Several common objects that are made of polymeric materials: plastic tableware (spoon, fork, and knife), billiard balls, a bicycle helmet, two dice, a lawnmower wheel (plastic hub and rubber tire), and a plastic milk carton.
A composite is composed of two (or more) individual materials, which come from the categories discussed above—viz., metals, ceramics, and polymers. The design goal of a composite is to achieve a combination of properties that is not displayed by any single material, and also to incorporate the best characteristics of each of the component materials. A large number of composite types exist that are represented by different combinations of metals, ceramics, and polymers. Furthermore, some naturally-occurring materials are also considered to be composites—for example, wood and bone. However, most of those we consider in our discussions are synthetic (or man-made) composites.
One of the most common and familiar composites is fiberglass, in which small glass fibers are embedded within a polymeric material (normally an epoxy or polyester). The glass fibers are relatively strong and stiff (but also brittle), whereas the polymer is ductile (but also weak and flexible).
Thus, the resulting fiberglass is relatively stiff, strong, (Figures 1.2 and 1.3) flexible, and ductile. In addition, it has a low density (Figure 1.1).
Another of these technologically important materials is the “carbon fiber reinforced polymer” (or “CFRP”) composite—carbon fibers that are embedded within a polymer. These materials are stiffer and stronger than the glass fiber-reinforced materials (Figures 1.2 and 1.3), yet they are more expensive. The CFRP composites are used in some aircraft and aerospace applications, as well as high-tech sporting equipment (e.g., bicycles, golf clubs, tennis rackets, and skis/snowboards).
Materials that are utilized in high-technology (or high-tech) applications are sometimes termed advanced materials. By high technology we mean a device or product that operates or functions using relatively intricate and sophisticated principles; examples include electronic equipment (camcorders, CD/DVD players, etc.), computers, fiber-optic systems, spacecraft, aircraft, and military rocketry. These advanced materials are typically traditional materials, whose properties have been enhanced,
and, also newly developed, high-performance materials. Furthermore, they may be of all material types (e.g., metals, ceramics, polymers), and are normally expensive.
Advanced materials include semiconductors, biomaterials, and what we may term “materials of the future” (that is, smart materials and nanoengineered materials), which we discuss below. The properties and applications of a number of these advanced materials—for example, materials that are used for lasers, integrated circuits, magnetic information storage, liquid crystal displays (LCDs), and fiber optics—are also discussed in subsequent chapters.
Semiconductors have electrical properties that are intermediate between the electrical conductors (viz. metals and metal alloys) and insulators (viz. ceramics and polymers)—Figure 1.5. Furthermore, the electrical characteristics of these materials are extremely sensitive to the presence of minute concentrations of impurity atoms, for which the concentrations may be controlled over very small spatial regions. Semiconductors have made possible the advent of integrated circuitry that has totally revolutionized the electronics and computer industries (not to mention our lives) over the past three decades.
Biomaterials are employed in components implanted into the human body for replacement of diseased or damaged body parts. These materials must not produce toxic substances and must be compatible with body tissues (i.e., must not cause adverse biological reactions). All of the above materials—metals, ceramics, polymers, composites, and semiconductors—may be used as biomaterials.
Smart (or intelligent) materials are a group of new and state-of-the-art materials now being developed that will have a significant influence on many of our technologies. The adjective “smart” implies that these materials are able to sense changes in their environments and then respond to these changes in predetermined manners—traits that are also found in living organisms. In addition, this “smart” concept is being extended to rather sophisticated systems that consist of both smart and traditional materials. Components of a smart material (or system) include some type of sensor (that detects an input signal), and an actuator (that performs a responsive and adaptive function). Actuators may be called upon to change shape, position, natural frequency, or mechanical characteristics in response to changes in temperature, electric fields, and/or magnetic fields. Four types of materials are commonly used for actuators: shape memory alloys, piezoelectric ceramics, magnetostrictive materials, and electrorheological/magnetorheological fluids. Shape memory alloys are metals that, after having been deformed, revert back to their original shapes when temperature is changed. Piezoelectric ceramics expand and contract in response to an applied electric field (or voltage); conversely, they also generate an electric field when their dimensions are altered. The behavior of magnetostrictive materials is analogous to that of the piezoelectric, except that they are responsive to magnetic fields. Also, electrorheological and magnetorheological fluids are liquids that experience dramatic changes in viscosity upon the application of electric and magnetic fields, respectively.
Materials/devices employed as sensors include optical fibers, piezoelectric materials (including some polymers), and microelectromechanical devices (MEMS).
For example, one type of smart system is used in helicopters to reduce aerodynamic cockpit noise that is created by the rotating rotor blades. Piezoelectric sensors inserted into the blades monitor blade stresses and deformations; feedback signals from these sensors are fed into a computer-controlled adaptive device, which generates noise-canceling antinoise.
![]()
![]()
Until very recent times the general procedure utilized by scientists to understand the chemistry and physics of materials has been to begin by studying large and complex structures, and then to investigate the fundamental building blocks of these structures that are smaller and simpler. This approach is sometimes termed “top_down” science. However, with the advent of scanning probe microscopes which permit observation of individual atoms and molecules, it has become possible to manipulate and move atoms and molecules to form new structures and, thus, design new materials that are built from simple atomic-level constituents (i.e., “materials by design”). This ability to carefully arrange atoms provides opportunities to develop mechanical, electrical, magnetic, and other properties that are not otherwise possible. We call this the “bottom-up” approach, and the study of the properties of these materials is termed “nanotechnology”; the “nano” prefix denotes that the dimensions of these structural entities are on the order of a nanometer
(10-9 m)—as a rule, less than 100 nanometers (equivalent to approximately 500 atom diameters). One example of a material of this type is the carbon nanotube, discussed in Section 12.4. In the future we will undoubtedly find that increasingly more of our technological advances will utilize these nanoengineered materials.
Source: https://uotechnology.edu.iq/dep-laserandoptoelec-eng/branch/lectures/solid%20state/chapter%201%20classification%20of%20materail.pdf
Web site to visit: https://uotechnology.edu.iq/
Author of the text: indicated on the source document of the above text
If you are the author of the text above and you not agree to share your knowledge for teaching, research, scholarship (for fair use as indicated in the United States copyrigh low) please send us an e-mail and we will remove your text quickly. Fair use is a limitation and exception to the exclusive right granted by copyright law to the author of a creative work. In United States copyright law, fair use is a doctrine that permits limited use of copyrighted material without acquiring permission from the rights holders. Examples of fair use include commentary, search engines, criticism, news reporting, research, teaching, library archiving and scholarship. It provides for the legal, unlicensed citation or incorporation of copyrighted material in another author's work under a four-factor balancing test. (source: http://en.wikipedia.org/wiki/Fair_use)
The information of medicine and health contained in the site are of a general nature and purpose which is purely informative and for this reason may not replace in any case, the council of a doctor or a qualified entity legally to the profession.
The texts are the property of their respective authors and we thank them for giving us the opportunity to share for free to students, teachers and users of the Web their texts will used only for illustrative educational and scientific purposes only.
All the information in our site are given for nonprofit educational purposes