You have already got acquainted with two groups of metal constructional materials: these are plain carbon steels and cast irons. Besides carbon steels, there is very big variety of grades of alloyed steels. This results from the fact that the steel is a unique material: it combines high rigidity (the modulus of elasticity E = 2.1·105 MPa), static and cyclic strength with high enough reliability of structures in service. It is possible to receive the necessary combination of mechanical properties both at the expense of change in steel composition (carbon and alloying elements percentage) and by a heat treatment.
Other constructional materials (alloys based on aluminium, magnesium, titanium, copper, and polymers) concede steels in rigidity, strength, reliability.
The word “alloying” originates for verb to alloy, means melt together. Alloying elements are added for increasing structural strength of steel and giving to it special service characteristics.
For metal-works and the parts working under severe conditions, the alloyed steels are used, instead of carbon ones.
First of all inexpensive and not scarce elements are applied, especially for bulk articles. They are manganese, silicon, chromium. In addition steels are alloyed with the elements restraining growth of grain such as titanium, vanadium, boron. But it is necessary to apply much more expensive and scarce nickel, molybdenum, tungsten, and niobium for especially responsible parts.
According to a quantity of alloying elements steels are subdivided onto three classifications: low-alloy steels (these contain no more than 2.5 % of alloying elements), medium-alloy steels (with content of alloying elements from 2.5 to 10 %), and high-alloy steels (more than 10 % of alloying additives).
Low-alloy steels are widely applied in building whereas the mechanical engineering uses alloyed ones mostly. All high-alloy steels have a special purpose: corrosion resistant steels, heat resistant steels, nonmagnetic etc.
1. The basic structural component of constructional steels is ferrite (up to 90 %). Therefore alloying elements should strengthen ferrite first of all. (Cementite does not require hardening.) Silicon, manganese and nickel give the maximum hardening of ferrite (Fig. 1, a). These elements do not form their own carbides therefore they are present in a solid solution only. Silicon besides is dissolved as interstitial impurity that gives especially effective hardening.
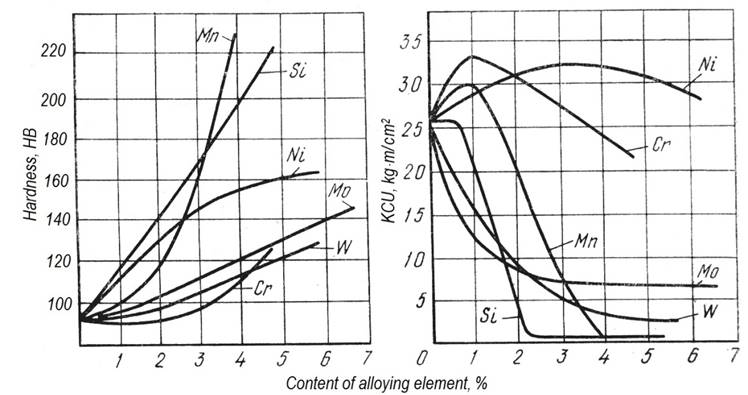
а b
Figure 1Influence of alloying elements on mechanical properties of steel:
а – steel hardness after annealing; b – impact toughness
Alloying elements do not influence plasticity almost, but reduce impact strength being added more than 1 % (Fig. 1, b). From this it follows that alloying should be rational: it is necessary to add the least necessary quantity of each element, and preferably use a complex of alloying elements, instead of any one.
2. Alloying elements raise critical temperatures of steel therefore ranges of temperature for alloyed steels heat treatment are higher, than for plain carbon steels.
3. Alloying elements reduce a critical cooling rate for steel quenching. For austenite decomposition carbon diffusion is necessary. In the structure of plain carbon steels small atoms of carbon move in the iron crystalline lattice easily. But for alloying austenite decomposition the diffusion of alloying elements should pass. Their atoms are comparable with atoms of iron by the size, and the diffusion rate is much less than one for carbon. Overcooled austenite becomes more stable (Fig. 2).
Relationship for critical cooling rates of various steels shown in Fig. 2:
Vcr3 <<Vcr2 <<Vcr1.
Therefore the alloyed steels can be quenched with the smaller rate of cooling than carbon ones; softer environments are possible to use. It reduces deformation of work-pieces and danger of cracks formation.
The diagram shown in Fig. 2, d is for austenitic steels; they have point Ms <0 °С and consequently cannot be quenched by any cooling rate.
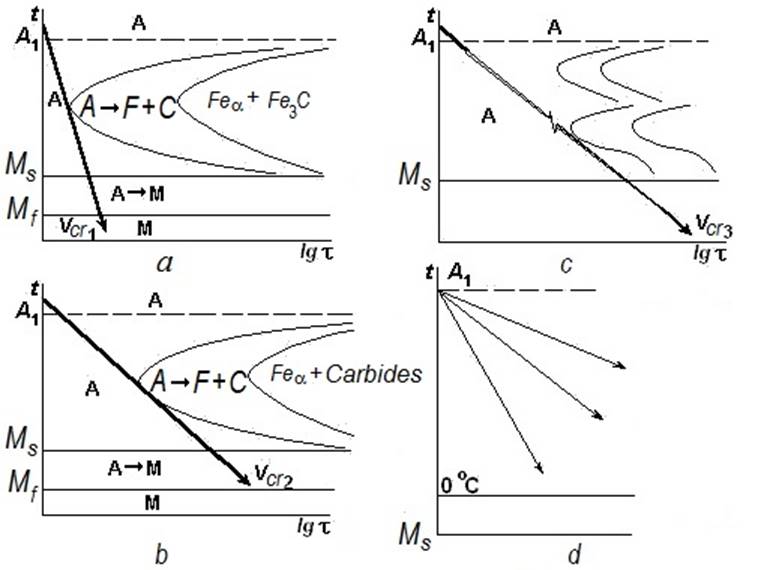
Figure 2Diagrams of isothermal austenite transformation:
а – plain carbon steel (cooling in water); b – low-alloy steel (cooling in oil); c – high-alloy steel (cooling in air); d – steel alloyed by Ni or Mn (martensite cannot be obtained)
4. Reducing critical cooling rate the alloying elements increase hardness penetration of steels, especially if alloying is complex. There are some steels in which martensite structure can be produced in any section. Therefore the large responsible machine parts and the small parts of complex shape are made of the alloyed steels only.
5. Alloying elements refine steel grain. It promotes increase in strength, plasticity, impact strength, and reduces the temperature of ductile-to-brittle transition. The tenth shares of percent of vanadium, titanium, niobium, and zirconium especially strongly reduce average grain size as this elements form carbides hardly soluble in austenite.
6. Alloying elements raise martensite’s resistance to tempering. After tempering at the same temperature the alloyed steels will be stronger (alloying elements strengthen ferrite and form carbide particles which are smaller, than cementite).
7. Alloying elements give to steels special physical and chemical properties: corrosion resistance, high elasticity, wear resistance, ability not to be oxidised upon heating, special magnetic properties, etc.
In the beginning of grade name the two digits specify the carbon content in hundredths of a percent by weight. They are followed by the letters designating alloying elements. Each letter is followed by digit designating the content of this alloying element in percent by weight. For the content of 1–1.5 % the figure is not put. The letter «А» in the end of grade name means the lowered content of harmful impurity.
А – nitrogen (within the grade name) К – cobalt Т – titanium
Б – niobium Н – nickel Ф – vanadium
В – tungsten M – molybdenum Х – chromium
Г – manganese П – phosphorus Ц – zirconium
Д – copper Р – boron Ч – rare-earth metal
Е – selenium С – silicon Ю – aluminium
«А» in the beginning of grade name means free-cutting steel (for high-speed processing by automatic machines).
These steels contain no more than 0.22 wt% C and a small amount of not scarce alloying elements: Mn, Si, Cr, Ni, Cu, V, and Ti. Nickel and copper are added because they lower the temperature of ductile-to-brittle transition and increase corrosion resistance.
This classification includes such grades as 09Г2С, 14Г2, 15ГФ, 15ХСНД, 10ХНДП. The low carbon content is necessary for good weldability. They are stronger than plain carbon steels and have the low temperature of ductile-to-brittle transition. Their application allows saving metal in building designs.
The steels are supplied in the form of sheets and high-quality shapes, sometimes in the air-hardened (normalized) state.
Steel of grade 16Г2АФ contains vanadium and nitrogen, therefore its structure includes vanadium carbonitride that promotes to preserve very fine grain size (6–8 μm in diameter) and to keep high impact strength at the low temperature.
Application examples:
Steel of grades 15ХСНД and 16Г2АФ is used for motor transport bridges; welded tanks and capacities are made of steel 10Г2С1 or 12Г2СМФ; pipes of the big diameter are produced of steel 17ГС in the air-hardened condition; for less responsible pipes hot-rolled steel is applied.
All steels mentioned in this group of Russian classification may be classified as high-strength low-alloy steels of AISI/SAE steel grades system.
Case-hardening steels
These contain 0.15–0.25 wt% C, the total content of alloying elements is no more than 6–7 %.
After cementation, quenching and low tempering the hardened layer should have hardness 58–62 HRC, and a core of a work-piece is 30–42 HRC.
For small parts of the simple shape chromium steels are applied: 15Х, 20Х, 20ХФ. They are stronger than the similar carbon steels.
For the large, responsible parts working under dynamic loadings, steels with nickel are used: 12ХН3А, 12Х2Н4А.
The steel of grade 18Х2Н4ВА is hardened practically in any section; any cooling rate gives the martensite structure. It has the temperature of ductile-to-brittle transition equal to –80 °С: above this temperature a fracture is shear. It is intended for the large hard loaded details (for example, crankshaft of the diesel locomotive engine).
For medium loaded cogwheels in the automobile, tractor and machine-tool industries steels 18ХГТ, 25ХГТ, 25ХГМ are applied.
Heat-hardenable steels
These are steels for very wide range of machine components: cranked shafts, axes, stems, connecting rods, parts of turbines and compressors. They contain 0.3–0.5 wt % C.
Heat treatment includes quenching and high tempering (such a combination is called a heat refining, or a toughening) Structure after heat treatment is sorbite. Steels have a high yield strength, fatigue strength, impact strength, and resistance to crack growth. Mechanical properties depend on heat treatment, especially temperature of tempering is important.
The medium loaded parts working without the big dynamic loadings are usually made of steels 30Х, 40Х, 50Х, 40ХФА.
The responsible welded designs are produced of 20ХГС, 25ХГС, 30ХГС. They have high strength and good weldability.
Steels with nickel possess high hardness penetration, yield strength, and impact strength. The following grades are widely spread in Russia: 40ХН, 50ХН, 40ХН2МА. They withstand to dynamic loadings and do not become brittle at the subzero temperatures. They are applied for shaft and rotors of turbines, parts of compressors and reducers.
Free-cutting steels
These are steels for mass production of small irresponsible machine parts (fixture, etc.). They allow processing steel by cutting with a great speed, in the same time increasing the tool life and raising quality of a surface.
Steels А12, А20, А40Г contain the raised quantity of sulphur (up to 0.3 wt %), phosphorus (up to 0.05 wt %), and manganese. The matter is that manganese sulphides promote formation of a short and brittle shaving and lubricate a cutter. Phosphorus in addition increases hardness and make steel more brittle. In mass production it is important, that the chips was fine and shovelling (element chips instead of continuous ones), therefore, it is necessary even to decrease mechanical properties.
Free cutting steels are alloyed also by Pb (АС14), Se, Te, and Ca. All these additives lower structural strength of steel, but speed of cutting increases by 40 % whereas the tool life stays invariable. Or it is possible to increase 2–7 times the tool life while cutting speed is constant.
Spring steels
The material for elastic elements should have the high modulus of elasticity E, elastic strength σE and fatigue limit σ-1. (It is necessary to remember that exactly the elastic modulus defines rigidity of a material and its resistance to elastic deformation: E = σ / δ.)
Spring steels have the raised carbon content in comparison with other constructional steels: 0.5–0.7 wt %.
Heat treatment consists of quenching and tempering at 420–500 °С, or isothermal quenching is possible. The first treatment gives temper troostite, while the second produces structure of bottom bainite.
Small springs are made of carbon steels grades from 65 to 85 and also 60Г–70Г containing increased amount of manganese. For more large springs and spring hangers (thickness up to 18 mm) steels 55С2, 60С2, or 70С3А are used.
For responsible springs and car spring hangers steels of such grades as 50ХФА and 50ХГФА are applied.
The large hard loaded, especially responsible springs require such steels as 60С2ХА and 60С2Н2А.
A choice of alloying elements is explained by the following: silicon strengthens ferrite, manganese increases hardness penetration, chromium and vanadium reduce the grain growth in the process of heating, nickel allows working in the conditions of dynamic loadings.
In order to increase a fatigue limit ready springs and spring sheets are hardened by shot-blasting processing. In a surface layer compressive stresses are produced that raises σ-1 1.5–2 times.
Ball bearing steel
Rolling bearings are responsible parts of machine tools, cars, electric motors which define their accuracy and productivity. Balls or rollers sliding on external and internal rings are affected abrasion and contact wear. Therefore, the main properties of ball-bearing steels are high hardness, wear resistance and resistance to contact fatigue.
In order to assure these properties high-carbon chromium steel ШХ15 (1 wt % C and 1.5 wt % Cr) is necessary. For large rings steel ШХ15СГ (1 % C, 1.5 % Cr, 0.5 % Si and 1.5 % Mn) is used because it has more depth of the hardening zone.
A typical cause of the bearing failure is fructure of balls or rollers and fatigue shelling-out of their surfaces. Therefore, it is very important that the steel does not contain nonmetallic inclusions which play a role of crack arising. For this purpose electroslag and vakuum-arc remelting are applied; steel grades are designated as ШХ15-Ш, ШХ15-ВД.
Heat treatment consists of quenching in oil and low tempering. Steel structure after heat treatment represents martensite and fine carbide particles. Hardness of rings and rollers should make 60–65 HRC, hardness of balls is 62–66 HRC.
A steel grade ШХ15 corresponds to SAE 52100.
Corrosion resistant steels
Corrosion is a destruction of metals under the influence of environment. In corrosion resistant steels this process develops with small rate.
Electrochemical corrosion goes in solutions of electrolytes: in damp soil, atmosphere, in sea and river water. Metal is dissolved because of a corrosion current between more and less electropositive sites of an alloy.
There is a group of electropositive metals, such as Pt, Au, Ag, Cu (and also Sn and Pb in many environments). These are not subject to electrochemical corrosion. But also electronegative metals can resist to corrosion too, for example Ti, Al, and Cr. They passivate, i.e. form very thin oxide films on a surface, which serves as a protective barrier to further corrosion.
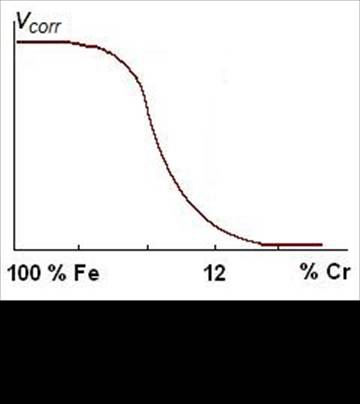 In corrosion resistant steels the basic alloying element is chromium. Its quantity should exceed 12.5 wt% as only such a content gives a continuous film of oxide Cr2O3 on a steel surface, and corrosion rate sharply decreases (Fig. 3).
In corrosion resistant steels the basic alloying element is chromium. Its quantity should exceed 12.5 wt% as only such a content gives a continuous film of oxide Cr2O3 on a steel surface, and corrosion rate sharply decreases (Fig. 3).
At normal temperature in a damp air, in the water, in some acids chromium steels are used. Examples of application are:
Figure 3 Influence
of chromium content on
corrosion rate of steel
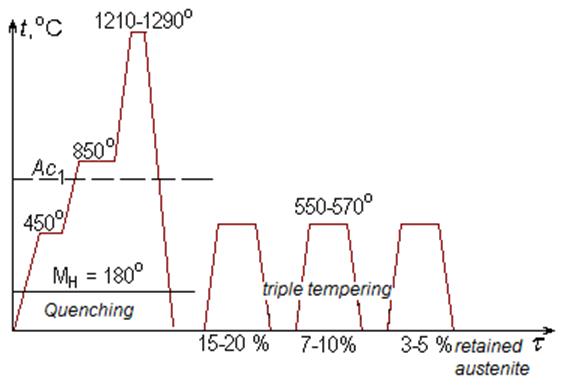
Figure 4Scheme of heat treatment operations for high-speed steels
Heating is carried out with one or two preheating in the fused salts for prevention of cracking and tool deformation because of low heat conductivity of steels. Tools of the simple shape are cooled in oil, and difficult ones – in solutions of salts (KNO3) at 250–400 °С.
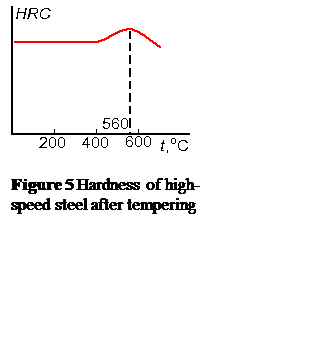
After hardening the structure of high-speed steel consists of high-alloyed martensite (0.3–0.4 % of carbon), the excessive carbides that failed to dissolve upon heating, and 20–30 % of retained austenite. The last reduces hardness and cutting properties of the tool and its presence it is undesirable.
In the course of repeated tempering disperse carbides precipitate out of retained austenite, the content of alloying elements in austenite decreases, and it undergoes to martensite transformation. A triple tempering at 550–570 °С within 45–60 minutes is usually applied. The number of tempering operations can be reduced due to subzero treatment after quenching which results in the content of retained austenite decreasing. But this type of processing is suitable for tools of the simple shape only. Hardness of high-speed steel after quenching is 62–63 HRC, and after tempering it increases to 63–65 HRC (Fig. 5).
 For the further increase in hardness, wear resistance and corrosion resistance of cutting tools surfaces the following processing can be applied: cyanide hardening, nitriding, steam treatment and other technologies of face hardening. They are carried out after the finish heat treatment, grinding and sharpening of tools.
For the further increase in hardness, wear resistance and corrosion resistance of cutting tools surfaces the following processing can be applied: cyanide hardening, nitriding, steam treatment and other technologies of face hardening. They are carried out after the finish heat treatment, grinding and sharpening of tools.
SAE International, as a standards organization, maintains several alloy numbering systems, one of which, for steel grades, is the SAE steel grades system.
In the 1930s and 1940s the American Iron and Steel Institute (AISI) and SAE were both involved in efforts to standardize such a numbering system for steels. These efforts were similar and overlapped significantly. For several decades the systems were united into a joint system designated the AISI/SAE steel grades. In 1995 the AISI turned over future maintenance of the system to SAE because the AISI never wrote any of the specifications.
Today steel quotes and certifications commonly make reference to both SAE and AISI, not always with precise differentiation. For example, in the alloy/grade field, a cert might say “4140”, “AISI 4140”, or “SAE 4140”, and in most light-industrial applications any of the above is accepted as adequate, and considered equivalent, for the job at hand, as long as the specific specification called out by the designer (for example, “4140 bar per ASTM-A108” or “4140 bar per AMS 6349”) is certified to on the certificate. The alloy number is simply a general classifier, whereas it is the specification itself that narrows down the steel to a very specific standard.
The SAE steel grade system's correspondence to other alloy numbering systems, such as the ASTM-SAE unified numbering system (UNS), can be seen in cross-referencing tables (including the ones given below).
The AISI system used a letter prefix to denote the steelmaking process. The prefix “C” denoted open-hearth furnace, electric arc furnace or basic oxygen furnace, while “E” denotes electric arc furnace steel. A letter “L” within the grade name indicates lead as an added ingredient; for example, 12L14 is a common grade that is 1214 with lead added for machinability.
Carbon steels and alloy steels are designated by a four digit number, where the first digit indicates the main alloying element(s), the second digit indicates the secondary alloying element(s), and the last two digits indicate the amount of carbon, in hundredths of a percent by weight. For example, a 1060 steel is a plain-carbon steel containing 0.60 wt% C.
An “H” suffix can be added to any designation to denote hardenability is a major requirement. The chemical requirements are loosened but hardness values defined for various distances on a Jominy test.
Table 1 Major classifications of steel
SAE designation |
Type |
1xxx |
Carbon steels |
2xxx |
Nickel steels |
3xxx |
Nickel-chromium steels |
4xxx |
Molybdenum steels |
5xxx |
Chromium steels |
6xxx |
Chromium-vanadium steels |
7xxx |
Tungsten steels |
8xxx |
Nickel-chromium-molybdenum steels |
9xxx |
Silicon-manganese steels |
Carbon and alloy steel grades |
|
SAE designation |
Type |
Carbon steels |
|
10xx |
Plain carbon (Mn 1.00% max.) |
11xx |
Resulfurized |
12xx |
Resulfurized and rephosphorized |
15xx |
Plain carbon (Mn 1.00–1.65%) |
Manganese steels |
|
13xx |
Mn 1.75% |
Nickel steels |
|
23xx |
Ni 3.50% |
25xx |
Ni 5.00% |
Nickel-chromium steels |
|
31xx |
Ni 1.25%; Cr 0.65%, or 0.80% |
32xx |
Ni 1.25%; Cr 1.07% |
33xx |
Ni 3.50%; Cr 1.50%, or 1.57% |
34xx |
Ni 3.00%; Cr 0.77% |
Molybdenum steels |
|
40xx |
Mo 0.20%, 0.25%, or Mo 0.25% and S 0.042% |
44xx |
Mo 0.40%, or 0.52% |
Chromium-molybdenum (chromoly) steels |
|
41xx |
Cr 0.50%, 0.80%, or 0.95%; Mo 0.12%, 0.20%, 0.25%, or 0.30% |
Nickel-chromium-molybdenum steels |
|
43xx |
Ni 1.82%; Cr 0.50–0.80%; Mo 0.25% |
43BVxx |
Ni 1.82%; Cr 0.50%; Mo 0.12%, or 0.35%; V 0.03% min |
47xx |
Ni 1.05%; Cr 0.45%; Mo 0.20%, or 0.35% |
81xx |
Ni 0.30%; Cr 0.40%; Mo 0.12% |
81Bxx |
Ni 0.30%; Cr 0.45%; Mo 0.12%; and added boron |
86xx |
Ni 0.55%; Cr 0.50%; Mo 0.20% |
87xx |
Ni 0.55%; Cr 0.50%; Mo 0.25% |
88xx |
Ni 0.55%; Cr 0.50%; Mo 0.35% |
93xx |
Ni 3.25%; Cr 1.20%; Mo 0.12% |
94xx |
Ni 0.45%; Cr 0.40%; Mo 0.12% |
97xx |
Ni 0.55%; Cr 0.20%; Mo 0.20% |
98xx |
Ni 1.00%; Cr 0.80%; Mo 0.25% |
Nickel-molybdenum steels |
|
46xx |
Ni 0.85%, or 1.82%; Mo 0.20%, or 0.25% |
48xx |
Ni 3.50%; Mo 0.25% |
Chromium steels |
|
50xx |
Cr 0.27%, 0.40%, 0.50%, or 0.65% |
50xxx |
Cr 0.50%; C 1.00% min |
50Bxx |
Cr 0.28%, or 0.50%; and added boron |
51xx |
Cr 0.80%, 0.87%, 0.92%, 1.00%, or 1.05% |
51xxx |
Cr 1.02%; C 1.00% min. |
51Bxx |
Cr 0.80%; and added boron |
52xxx |
Cr 1.45%; C 1.00% min. |
Chromium-vanadium steels |
|
61xx |
Cr 0.60%, 0.80%, 0.95%; V 0.10%, or 0.15% min. |
Tungsten-chromium steels |
|
72xx |
W 1.75%; Cr 0.75% |
Silicon-manganese steels |
|
92xx |
Si 1.40%, or 2.00%; Mn 0.65%, 0.82%, or 0.85%; Cr 0.00%, or 0.65% |
High-strength low-alloy steels |
|
9xx |
Various SAE grades |
xxBxx |
Boron steels |
xxLxx |
Leaded steels |
Copper is a heavy metal (γ = 8.9 g/cm3) with FCC lattice; it does not have any polymorphic transformations. Temperature of fusion is 1083 °C. It is possible to call copper “the most colourful” metal: its surface is red, and a fracture is pink.
Pure copper is applied more often in the electrical engineering and electronics. Copper possesses high electrical conductivity therefore it is used as a current conductor (buses, cable cores, windings of electric motors, contacts).
The highest heat conductivity of copper allows making water cooled crucibles, crystallizers, pallets, ingot moulds etc.
Copper shows corrosion resistance in atmosphere, sea, river and tap water, in other excited environments.
Technological properties of copper are not so good: it is very ductile and can be easily processed by pressure, but it has bad machinability. Foundry properties are low (copper gives notable shrinkage); copper is hard to weld, but easy to solder.
Copper has low strength: from 160 MPa in a cast condition to 240 after hot deformation. Nevertheless, the wire drawn is cold worked up to σT = 450 MPa.
Copper is supplied in the form of rolled shapes: sheets, bars, pipes and wire.
Copper alloys represent solid solutions based on copper. They are both stronger and more plastic than pure copper. Strength of copper alloys is equal to strength of annealed low-carbon steel (450 and 500 MPa accordingly).
All copper alloys are subdivided into two groups: brass and bronze.
Brass
Brasses are alloys of copper and zinc. If a brass does not contain any other alloying element except for zinc, it is a simple brass; if there are also other additives in its composition, an alloy is called a special brass.
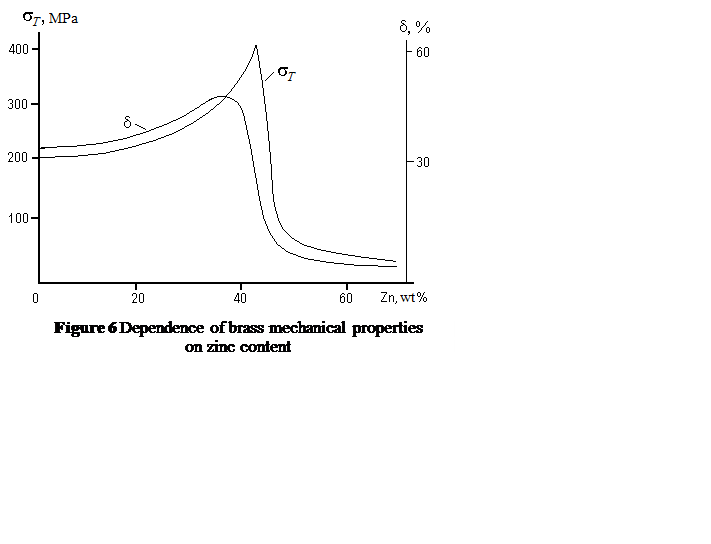
Zinc is dissolved in copper up to 39 % wt., forming α phase which is substitutional solid solution. Addition Zn over 39 % wt. produces β phase CuZn with BCC lattice (the ordered solid solution based on chemical compound). This gives higher strength but accompanied by sharp fall in ductility (Fig. 6).
Brasses with zinc content no more than 45 % wt. are applied, as at higher concentration the structure consists of brittle β phase only.
 Single-phase, or α brasses are ductile metals; the products are made by cold deformation processes. These include any parts feasible by sheet-metal die forging, as well as wire, tapes, radiator tubes, cartridge cases, electro-technical details.
Single-phase, or α brasses are ductile metals; the products are made by cold deformation processes. These include any parts feasible by sheet-metal die forging, as well as wire, tapes, radiator tubes, cartridge cases, electro-technical details.
Additives of nickel and iron raise strength of brass up to 550 MPa.
The brass keeps plasticity and toughness upon temperatures below 0 °C.
Bronze
Bronzes are alloys of copper with all other elements, except zinc. In their title the adjectives specifying the second component are used. Depending on composition and structure after machining strength of bronzes can be raised from 200 to 750 MPa. Bronzes are subdivided onto aluminium bronzes, tin bronzes, silicon bronzes, beryllium bronze etc. In Russian grade system bronzes are labelled by letters «Бр» followed by the digits specifying alloying elements and their contents in % wt. For example:
БрОФ10–1 contains 10 % wt. Sn + 1 % wt. P, copper is the balance;
БрОЦС4–4–2.5 contains 4 % wt. Sn + 4 % wt. Zn + 2.5 % wt. Pb, copper is the balance;
БрС30 includes 30 % wt. Pb, copper is the balance;
БрКМц3–1 consists of 3 % wt. Si + 1 % wt. Mn, copper is the balance.
The simplest bronzes are tin ones, known since the Bronze Age. They, as well as other alloys, are subdivided onto wrought bronzes (< 10 % wt. Sn) and cast bronzes (> 10 % wt. Sn). Formerly bronzes gained the title depending on their purpose: monetary (4–10 % wt. Sn), gun (8–18 % wt. Sn), bell (20–30 % wt. Sn), and mirror (30–35 % wt. Sn) bronzes. Tin bronzes are distinguished with good casting characteristics, for example, small crystallization shrinkage; therefore it is possible to mould articles of a complex configuration. For the purpose of expensive tin saving zinc is added to bronzes; the amount of addition is that it completely dissolved in copper, forming a solid solution and thereby raising mechanical properties. For raise antifriction properties and the best machining property lead is added to tin bronzes (БрОЦС4–4–17). The cast tin bronzes are applied for the steam-and-water fixture as they possess a high corrosion resistance in water and in air.
Deformable bronzes have single-phase structure of a solid solution. After cold working by pressure, bronze is exposed to annealing at 600–700 ºС. They are more malleable and stronger, than cast bronzes. Besides, deformed tin bronzes possess high elastic properties; therefore they are used for springs and diaphragms in the electrical engineering and other areas.
Aluminium bronzes usually contain from 5 to 10 % wt. of aluminium. Mechanical and corrosion properties of these bronzes are higher, than ones for tin bronzes. As for brasses and tin bronzes, in the process of composition change, properties of aluminium bronzes vary also: hardness НВ, strength sT and plasticity d sweepingly grow with increase in aluminium content; then toughness and strength are downgraded because of the brittle second phase formation. Therefore in practice the two-phase bronzes containing no more than 11 % wt. of aluminium are applied. Two-phase bronzes have high strength up to 600 MPa and hardness above 100 НВ. Aluminium bronzes experience eutectoid transformation; hence, they can be quenched and age-hardened. Single-phase aluminium bronzes (БрА7) are more plastic, than two-phase, and refer to the wrought ones. They possess high strength and plasticity (sT = 400–450 MPa, d = 60 %).
Aluminium bronzes are alloyed by iron, nickel, manganese for elimination of casting defects and increase in mechanical properties after strengthening heat treatment (quenching and the subsequent age-hardening). For example, for bronze БрАЖН 10–4–4 hardness grows from 140–160 НВ to 400 НВ. Therefore, Al–Fe–Ni bronzes are used for fabrication the parts working on the wearing conditions: saddles of valves, guide bushes, parts of pumps and turbines, gear wheels, etc.
Silicon bronzes contain no more than 3 % wt. Si and substitute tin bronzes. They are plastic, corrosion resistant in some excited environments, and have a good weldability and solderability. For raise in hardness and strength silicon bronzes are alloyed by manganese and nickel with the subsequent heat treatment. These bronzes are used instead of more expensive tin ones for manufacture antifriction parts, and also they substitute beryllium bronzes for springs.
Lead bronzes (БрС30) possess high antifriction properties, high heat conductivity (under less than 300 °C). Lead bronzes have low strength, but they are very plastic, and resist to blows well. Therefore, these bronzes are applied for bearing liners working upon the big pressures and speeds.
Beryllium bronze (БрБ2) contain no more than 2–2.5 % wt. of beryllium. As beryllium produces a solid solution of variable solubility with copper, this bronze is applied only after strengthening heat treatment (quenching from 780 °С and age-hardening at 320 °С). Both strength and elastic properties thus rise: sT = 1300–1500 MPa, sE = 600–740 MPa. Besides, beryllium bronzes possess a high electrical conductivity; therefore they are applied for springs in the electric equipment, for elastic contacts etc. But high cost does not allow applying this bronze widely. It may be substitute by more low-cost titanic or titanic-chromium bronze. After heat treatment it has almost the same strength characteristics, but is more plastic and has high relaxation durability at the temperature below 400 °С. Therefore, elastic elements made of such bronze can work at higher temperature than ones made of beryllium bronzes.
Source: https://portal.tpu.ru/SHARED/k/KHVOROVA/eng/Teaching/Tab1/Metal_Structural_Materials.docx
Web site to visit: https://portal.tpu.ru/
Author of the text: indicated on the source document of the above text
If you are the author of the text above and you not agree to share your knowledge for teaching, research, scholarship (for fair use as indicated in the United States copyrigh low) please send us an e-mail and we will remove your text quickly. Fair use is a limitation and exception to the exclusive right granted by copyright law to the author of a creative work. In United States copyright law, fair use is a doctrine that permits limited use of copyrighted material without acquiring permission from the rights holders. Examples of fair use include commentary, search engines, criticism, news reporting, research, teaching, library archiving and scholarship. It provides for the legal, unlicensed citation or incorporation of copyrighted material in another author's work under a four-factor balancing test. (source: http://en.wikipedia.org/wiki/Fair_use)
The information of medicine and health contained in the site are of a general nature and purpose which is purely informative and for this reason may not replace in any case, the council of a doctor or a qualified entity legally to the profession.
The texts are the property of their respective authors and we thank them for giving us the opportunity to share for free to students, teachers and users of the Web their texts will used only for illustrative educational and scientific purposes only.
All the information in our site are given for nonprofit educational purposes